(2) Consider a container surrounded by adiabatic walls. The container is separated by a fixed and heat-conductive wall into two compartments Vi and V2. Using the condition to maximize entropy, show that the temperatures inside the two compartments are equal when the equilibrium is reached. Show this with and without using Lagrange multiplier.
(2) Consider a container surrounded by adiabatic walls. The container is separated by a fixed and heat-conductive wall into two compartments Vi and V2. Using the condition to maximize entropy, show that the temperatures inside the two compartments are equal when the equilibrium is reached. Show this with and without using Lagrange multiplier.
Related questions
Question
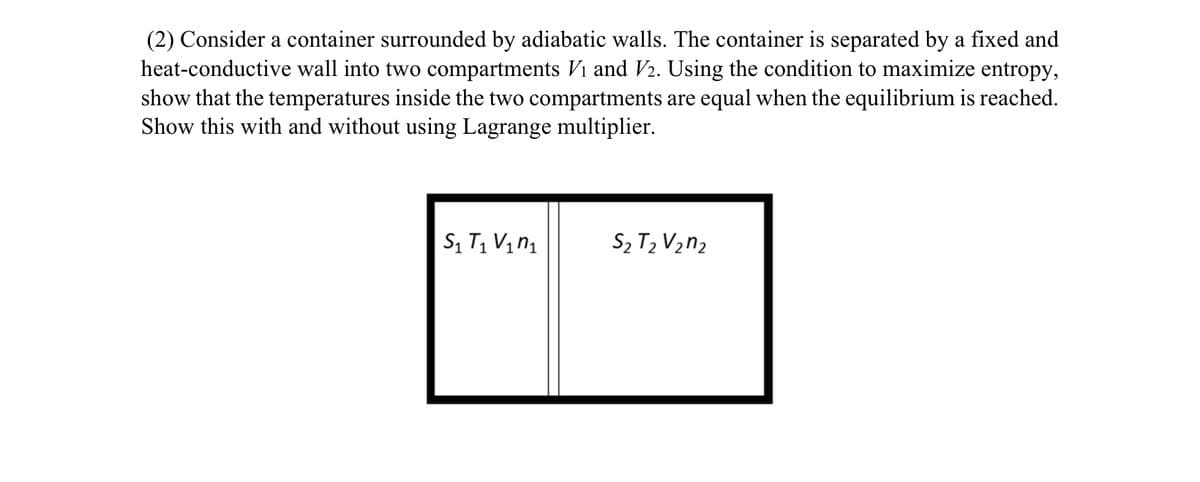
Transcribed Image Text:(2) Consider a container surrounded by adiabatic walls. The container is separated by a fixed and
heat-conductive wall into two compartments Vi and V2. Using the condition to maximize entropy,
show that the temperatures inside the two compartments are equal when the equilibrium is reached.
Show this with and without using Lagrange multiplier.
S₁ T₁ V₁₁
S₂ T₂ V₂n2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images
