4) For such a phase diagram, a) Do you expect the newly formed bonds (A-B) are stronger than the initial bonds or not? Briefly explain the reason. Is this solution formation reaction endothermic or exothermic? b) Then, is the activity coefficient greater than 1 or not? How would you expect change in volume and change in Enthalpy due to formation of the solution? c) Describe the function (interatomic bond parameter). Explain/compare the bond strength (newly formed A-B vs initial A- A and B-B) depending on sign of the 2-function. T L+α L A Хв B
4) For such a phase diagram, a) Do you expect the newly formed bonds (A-B) are stronger than the initial bonds or not? Briefly explain the reason. Is this solution formation reaction endothermic or exothermic? b) Then, is the activity coefficient greater than 1 or not? How would you expect change in volume and change in Enthalpy due to formation of the solution? c) Describe the function (interatomic bond parameter). Explain/compare the bond strength (newly formed A-B vs initial A- A and B-B) depending on sign of the 2-function. T L+α L A Хв B
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 114CP: Carbon tetrachloride (CCl4) and benzene (C6H6) form ideal solutions. Consider an equimolar solution...
Related questions
Question
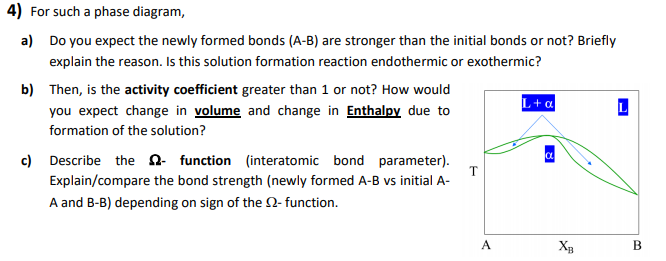
Transcribed Image Text:4) For such a phase diagram,
a) Do you expect the newly formed bonds (A-B) are stronger than the initial bonds or not? Briefly
explain the reason. Is this solution formation reaction endothermic or exothermic?
b) Then, is the activity coefficient greater than 1 or not? How would
you expect change in volume and change in Enthalpy due to
formation of the solution?
c) Describe the function (interatomic bond parameter).
Explain/compare the bond strength (newly formed A-B vs initial A-
A and B-B) depending on sign of the 2-function.
T
L+α
L
A
Хв
B
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning