(4) Starting from the equation of state for ideal gas (1 mol, pV = RT), show that the following (Joule's law) is true: au = 0 av T
Q: Power Calculation 1. A 50-kg marathon runner runs up the stairs to the top of the building at a…
A:
Q: Leo and Owen are working at a particle linear accelerator that is 414 meters long. A particle is…
A: Length of particle accelerator according to particle will be 189 m.Explanation:
Q: An object shot into the air follows the path given by (t) = (at, 1000+ bt - 4.9t²) m with t in…
A:
Q: Point-masses m, are located on the x-axis as shown. Find the moment M of the system about the origin…
A: The two mass m1 = 4 is located at 10 distances from origin.The two mass m2 = 12 is located at 30…
Q: A ▶2/56 The preliminary design for a rapid-transit sys- tem calls for the train velocity to vary…
A:
Q: A car with mass of 1108 kg accelerates from 0 m/s to 40.0 m/s in 10.0 s. Ignore air resistance. The…
A: Given mass of the car is m=1108kgThe initial speed is u=0m/sThe final speed is v=40m/sThe time is…
Q: closed path on a rectangular prism, an
A: A net is a 2D representation of a 3D shape that can be folded to form the original 3D shape. In this…
Q: = yuu0+Cv(T TO) w
A:
Q: 2. An isolated system consists of three identical, distinguishable atoms. Each atom can have energy…
A: To find the statistical weights (or multiplicities) of the macrostates with total energies of 0, ε,…
Q: A ball rolls onto the path of your car as you drive down a quiet neighborhood street. To avoid…
A:
Q: ground 6m A light shines from the top of a pole 10m high. You stand on the ground. away from the…
A: Speed is how fast an object moves. Speed is magnitude and velocity is how fast the object moves and…
Q: How to evaluate the 2 partial derivatives from the expression for Z?
A: The trignometric hyperbolic functions are defined as The derivatives of these function are
Q: Hello, I am stuck on the practice problem. I'm not sure I am doing it right. Could you help guide me…
A:
Q: Aq 2. Four charged particles are at the corners of a square of side a figure below. (Let A = 5, B =…
A:
Q: 2. The figure shows a pin-supported member, ABD, in static equilibrium. The mass of the member is…
A: The objective of this question is to calculate the support reactions at points B and D, and to…
Q: The cosmic microwave background radiation is strong evidence for the Big Bang theory because it: (a)…
A: The objective of the question is to understand why the cosmic microwave background radiation (CMBR)…
Q: 2. An object attached to the end of a spring is modeled by the ODE my" + by' + ky = Fexternal…
A:
Q: A chain 59 meters long whose mass is 24 kilograms is hanging over the edge of a tall building and…
A:
Q: a. b. Find the work done by the force of friction. Find the coefficient of friction.
A: The work energy theorem states that the work done on any object is equal to the difference between…
Q: Hypothesis:If I give a hearing test to 5 boys and 5 girls . Then the girls will be able to hear…
A: Hypothesis:If I give a hearing test to 5 boys and 5 girls . Then the girls will be able to hear…
Q: Let a planet revolve around a star, and the mass of the star decreases continuously due to radiation…
A:
Q: Task 8. Consider a unit cube of an isotropic solid occupying the region 0≤ x ≤ 1, 0≤ y ≤ 1, 0≤ z <1.…
A: Volume of a cube is given byV=xyz, where x,y and z are length elements.For a unit cube,…
Q: Two particles have charge -4.61 x 10-6 C and 7.75 x 10-6 C, respectively, and are 0.0134 meters…
A: To solve this problem, we'll use Coulomb's law, which states that the force (F) between two charges…
Q: Find the locations where the probability density has its maximum values for the wave function w(x) =…
A:
Q: The eigenvalues a
A:
Q: Occasionally, huge icebergs are found floating on the ocean's currents. Suppose one such iceberg is…
A:
Q: Sketch the spherical harmonics wavefunction with quantum numbers | = 1 and ml = 0
A: In quantum mechanics, there are four quantum numbers i.e. n,l,ml and ms.
Q: LASICISe Disk A, of weight 10 lb and radius r = 6 in., is at rest when it is placed in contact with…
A:
Q: A small boat is moving at a velocity of 1.73m/s when it is accelerated by a river current…
A:
Q: O. Add the following vectors: 5mE;7.5mS60°w;10mN; 3.5m35°N of W.
A: We will first draw the diagram showing the given vectors. We will then resolve the vectors into…
Q: ) A source of spherical waves of frequency f and pressure amplitude A at 1 meter is a distance d…
A:
Q: 8. Capacitor consists of a sphere surrounded by a concentric spherical shell (R1=5cm, R2=15cm)…
A:
Q: x Initially h is 268mm, Mass = 11 kg, k= 242 N/m and has unstretched length= 135 mm. Find the…
A:
Q: Block X Block Y VXY M M Figure 2. After the collision ock X slides along a horizontal surface with a…
A: here ,Block X slides along a horizontal surface with a speed vx toward block Y which is at rest and…
Q: 5) A Toy model of a + b → 1 + 2. In the toy model Mfi = 1 and unitless. The incoming and outgoing…
A:
Q: In an experiment, a small 20 W motor is used to lift a 100 g mass through a vertical distance. a) if…
A: The objective of this question is to calculate the potential energy gained by the mass and the…
Q: This function can be described by the x(t) = Age-Ytcos(wt) formula, where Ao is the initial…
A:
Q: A 6.53-kg block initially at rest is pulled to the right along a horizontal surface by a constant…
A:
Q: (2) [ Describe briefly why the following statements are wrong. (i) "Hot cup of coffee becoming cold…
A: I have written the description why both the statement are wrong. I hope this helps.Explanation:(i)…
Q: Jose is at rest in System S' that has a velocity u = +0.46c relative to system S, where Cisco is at…
A: Given : velocity of frame S' relative to frame S , u = 0.46c = 0.46 X 3X 108 m/s = 1.38X108 m/s…
Q: Bonus 2 (2 pts) When angular momentum f = 3, draw all possible angular momentum distributions in 3D,…
A: The minimum radius is 2π3h. Please see the figure in explanation for details.Explanation:Step 1:…
Q: A cart is moving at a constant absolute velocity Vcart = 5 km/h to the right. A high-speed jet of…
A: Velocity of the cart (Vcart) = 5 km/h to the right.Velocity of the water jet (Vjet ) = 15 km/h to…
Q: Three electrons form an equilateral triangle 1.99nm on each side. A proton is at the center of the…
A: Side of triangle, a= 1.99 nm
Q: An L-R-C series circuit has 0.300 H and C = 4.00 μF. Calculate the angular frequency of oscillation…
A: The objective of the question is to find the value of resistance that gives critical damping and the…
Q: Three negative point charges lie along a line as shown in ( Figure 1). Point P lies 6.00 cm from the…
A:
Q: Leo and Owen are working at a particle linear accelerator that is 414 meters long. A particle is…
A: The objective of the question is to calculate the length of the particle accelerator according to…
Q: A body of mass m is retarded by a force -Cv², where C is a con- stant and v is its speed. Find the…
A: A body of mass m is retarded by a force,It is initially moving at speed
Q: Calculate the power density (Pa) produced at a point (P) of 10Km distant from 80m long-wire antenna…
A: A power density of an antenna is expressed as the input power provided to the antenna divided by the…
Q: The elbow joint is flexed using the biceps brachii muscle, which re vertical as the arm moves in the…
A:
Q: Fourier transform (FT) of the 2-dimensional square function
A:
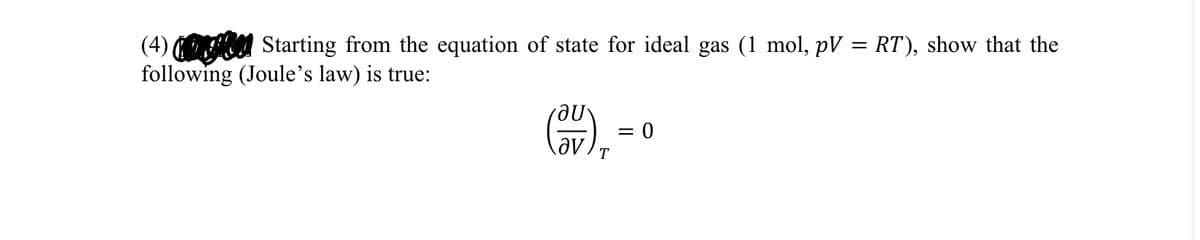
Trending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images
