CL Chem Chapter 17 continuation: Ksp Past AP Problems 2011 form B, question #1 Answer the following questions about the solubility and reactions of the ionic compounds M(OH)2 and MCO3, where M represents an unidentified metal. (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. (i) Calculate the molar concentration of OH (aq) in the saturated solution. (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of the solubility-product constant, K., for M(OH)2 at 25°C. sp (c) For the metal carbonate, MCO3, the value of the solubility-product constant, Kp is 7.4×10-14 at 25°C. On the basis of this information and your results in part (b), which compound, M(OH)2 or MCO, has the greater molar solubility in water at 25°C? Justify your answer with a calculation. (d) MCO, decomposes at high temperatures, as shown by the reaction represented below. MCO3(s) → MO(s) + CO₂(g) A sample of MCO3 is placed in a previously evacuated container, heated to 423 K, and allowed to come to equilibrium. Some solid MCO3 remains in the container. The value of K, for the reaction at 423K is 0.0012. (i) Write the equilibrium-constant expression for K, of the reaction. (ii) Determine the pressure, in atm, of CO2(g) in the container at equilibrium at 423 K. (iii) Indicate whether the value of AG° for the reaction at 423 K is positive, negative, or zero. Justify your answer. (SKIP this question for now! It's covered in chap 19)
CL Chem Chapter 17 continuation: Ksp Past AP Problems 2011 form B, question #1 Answer the following questions about the solubility and reactions of the ionic compounds M(OH)2 and MCO3, where M represents an unidentified metal. (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. (i) Calculate the molar concentration of OH (aq) in the saturated solution. (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of the solubility-product constant, K., for M(OH)2 at 25°C. sp (c) For the metal carbonate, MCO3, the value of the solubility-product constant, Kp is 7.4×10-14 at 25°C. On the basis of this information and your results in part (b), which compound, M(OH)2 or MCO, has the greater molar solubility in water at 25°C? Justify your answer with a calculation. (d) MCO, decomposes at high temperatures, as shown by the reaction represented below. MCO3(s) → MO(s) + CO₂(g) A sample of MCO3 is placed in a previously evacuated container, heated to 423 K, and allowed to come to equilibrium. Some solid MCO3 remains in the container. The value of K, for the reaction at 423K is 0.0012. (i) Write the equilibrium-constant expression for K, of the reaction. (ii) Determine the pressure, in atm, of CO2(g) in the container at equilibrium at 423 K. (iii) Indicate whether the value of AG° for the reaction at 423 K is positive, negative, or zero. Justify your answer. (SKIP this question for now! It's covered in chap 19)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 2ALQ: A friend asks the following: Consider a buffered solution made up of the weak acid HA and its salt...
Related questions
Question
Could I get a detailed explanation for c); d) i, ii, iii please
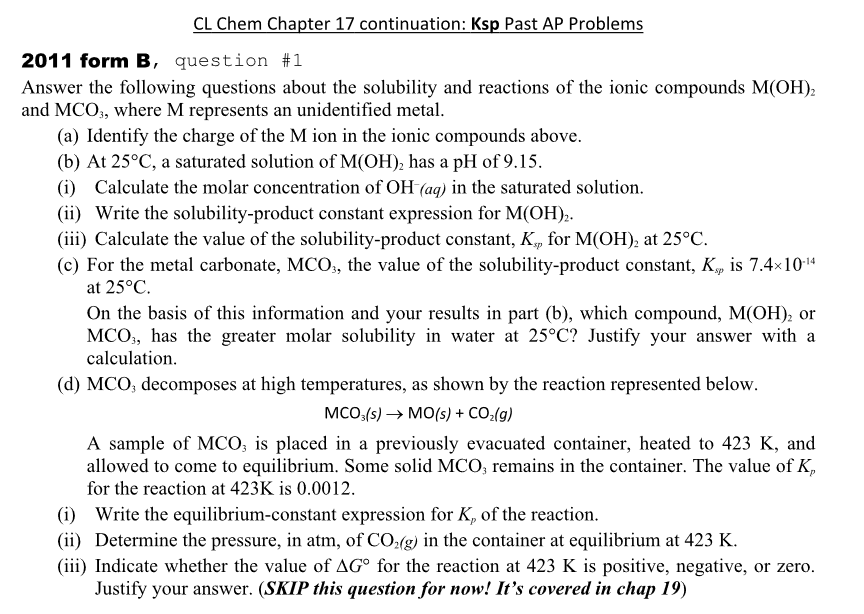
Transcribed Image Text:CL Chem Chapter 17 continuation: Ksp Past AP Problems
2011 form B, question #1
Answer the following questions about the solubility and reactions of the ionic compounds M(OH)2
and MCO3, where M represents an unidentified metal.
(a) Identify the charge of the M ion in the ionic compounds above.
(b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15.
(i) Calculate the molar concentration of OH (aq) in the saturated solution.
(ii) Write the solubility-product constant expression for M(OH)2.
(iii) Calculate the value of the solubility-product constant, K., for M(OH)2 at 25°C.
sp
(c) For the metal carbonate, MCO3, the value of the solubility-product constant, Kp is 7.4×10-14
at 25°C.
On the basis of this information and your results in part (b), which compound, M(OH)2 or
MCO, has the greater molar solubility in water at 25°C? Justify your answer with a
calculation.
(d) MCO, decomposes at high temperatures, as shown by the reaction represented below.
MCO3(s) → MO(s) + CO₂(g)
A sample of MCO3 is placed in a previously evacuated container, heated to 423 K, and
allowed to come to equilibrium. Some solid MCO3 remains in the container. The value of K,
for the reaction at 423K is 0.0012.
(i) Write the equilibrium-constant expression for K, of the reaction.
(ii) Determine the pressure, in atm, of CO2(g) in the container at equilibrium at 423 K.
(iii) Indicate whether the value of AG° for the reaction at 423 K is positive, negative, or zero.
Justify your answer. (SKIP this question for now! It's covered in chap 19)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning