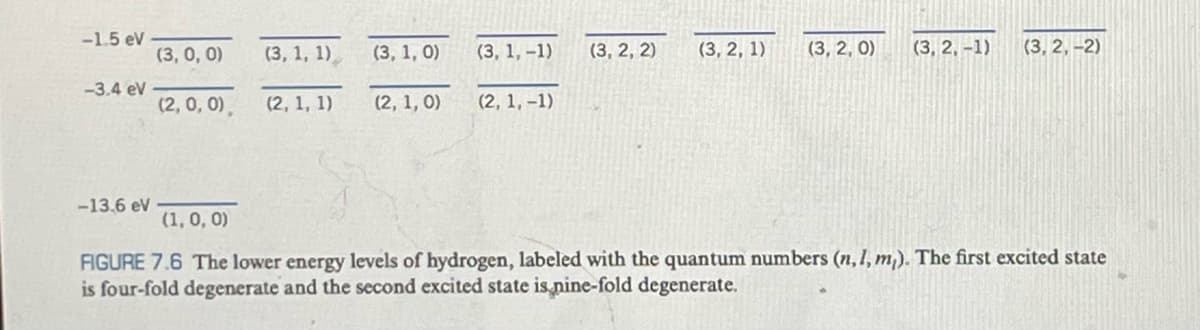
List the 16 possible sets of quantum numbers n, l, m, of the n = 4 level of hydrogen (as in Figure 7.6).
Q: acceleration a = (5) m/s² where t is in seconds, determine; A 250 mm (a)the angular velocity of…
A: Given Data:The acceleration of mass B is 5t4 m/s2.To Find:The angular velocity of the pulley.The…
Q: 5. A block 1.0 kg on a horizontal surface is attached to a spring (k = 24 N/m). The spring is…
A: Solution Given dataMass of block m=1.0kgSpring constant k=24N/mSpring compressed…
Q: Question 1: A sheet of glass (n=1.50) is coated with a 501-nm-thick layer of oil (n = 1.42). a) For…
A: Question 1:A sheet of glass (n=1.50) is coated with a 501-nm-thick layer of oil (n = 1.42).a) For…
Q: What is the capacitance of two square parallel plates 3.8 cm on a side that are separated by 1.4 mm…
A: Square plate of side Separation distance Dielectric constant Capacitance
Q: A wooden plank (3 m long) is supported at each end by two support beams. The plank has a mass of 55…
A:
Q: A cylinder of radius a and height L is centered about the z -axis and has a uniform polarization…
A: I have explained a detailed solution in the below steps.Explanation:Step 1:Step 2:Step 3:
Q: A small motor draws 120 J of electrical energy from the mains to lift a book of mass 1 kg through a…
A: The input energy is Ei=120JThe mass of the book is m=1kgThe vertical distance is h=8mThe time is…
Q: Public television station KQED in San Francisco broadcasts a sinusoidal radio signal at a power of…
A:
Q: he rigid rotor approximation
A:
Q: Harmonic dependence of the fields may be implmented via Ē(x,t) = Re [Ē() exp(-iwt)], where Ē() may…
A: Sure, here's a concise version of the answer:Time-averaged Poynting Vector:Given harmonic electric…
Q: Consider a cubic 3D infinite well. part a: How many different wave functions have the same energy as…
A: Therefore, the entirety of the observed degeneracy in this system can be attributed to…
Q: FM radio stations use radio waves with frequencies from 88.0 to 108 MHz to broadcast their signals.…
A: Cmin = 7.24 x 10-12 F Cmax = 10.9 x 10-12 FExplanation:Approach to solving the question: Detailed…
Q: At time t = 0 a 2150 kg rocket in outer space fires an engine that exerts an increasing force on it…
A:
Q: An electron is in a 3p state in the hydrogen atom: Part a: What is the probability density?
A: The objective of the question is to calculate the probability density of an electron in a 3p state…
Q: electrical flux
A:
Q: Part A A new car is tested on a 280-m-diameter track If the car speeds up at a steady 1.3 m/s², how…
A: Given diameter of the track is d=280mThe radius is r=d/2=280/2=140mThe tangential acceleration is…
Q: The leg and cast in the figure below weigh 173 N (w₁). Determine the weight w₂ and the angle a…
A: Given: Figure shows the weigh w1 , w2 ,110N ,40° and angle alpha.Where w1= 173NOur task is to…
Q: An aircraft has a radar transmitter that contains an LC circuit oscillating at 6.50 x 10 Hz. (a) For…
A:
Q: Using the definition and properties of the density function, show step by step derivation of…
A: Refer explanationExplanation:
Q: e? L-C circuit is
A:
Q: A 0.20-kg mass is attached to a string and then released from rest, swinging in an arc as a…
A: The objective of this question is to find the original height from which the mass was released. This…
Q: A hydrogen atom in an n=2 state absorbs a photon.What wavelength photons might be emitted by the…
A: Let's proceed with the calculations:1. \( \lambda_1 = \frac{{100 \times 3^2}}{{3^2 - 2^2}} =…
Q: A transparent optical material has a refractive index of 1.8. What is the Fresnel Reflectivity…
A: A transparent optical material has a refractive index of 1.8. What is the Fresnel Reflectivity…
Q: m M A small block of mass m = 720. g is compressing a spring of spring constant 323 N/m by 26.1 cm.…
A:
Q: Show that, using the Quantum Harmonic Oscillator model, the 3-4 vibrational transition is auowed…
A:
Q: Starting from rest, a person pedals a bicycle such that the angular acceleration of the wheels is a…
A:
Q: A 2.10 kg block is pulled up force on the block from th- Number i
A:
Q: A 1200 kg car is moving at 5.0 m/s EAST. It strikes an 1800 kg car at rest. The cars undergo an…
A:
Q: A convex mirror has a focal length of -26 cm. Find the magnification produced by the mirror when the…
A: (a) 0.703 no units(b) 0.553 no unitsExplanation:
Q: A semiconductor with 1013 donors/cm³. If you know that their donor level below the conduction band…
A:
Q: About how fast would you have to be driving toward a red (A = 685 nm) stoplight for it to appear…
A: Given Data:The wavelength of red light is λr=685 nm.The wavelength of red light is λr=532 nm.To…
Q: A plane mirror and a concave mirror (f = 5.80 cm) are facing each other and are separated by a…
A: The image distance form the concave mirror is 6.73 cm Thank you Let me know if you have any doubts…
Q: A rod of length L = 4.00 m with uniform charge of 9.50 nC/m is oriented along the y axis as shown in…
A:
Q: Consider n moles of a gas, initially confined within a volume V and held at temperature T. The gas…
A:
Q: In order to use the basic meter as an ohmmeter, a variable resistor and a DC voltage source, for…
A: The current on the ammeter = 1 mAThe variable resistance = RvThe unknown resistance = RxThe voltage…
Q: The starter motor of a car engine draws a current of 140 A from the battery. The copper wire to the…
A:
Q: QUESTION #7 An object is projected vertically up into the air from a balcony high above the ground.…
A: Given data:The time taken by the object to reach the maximum height is.t=3 s.The height difference…
Q: Charged particle motion Work done by an electromagnetic field by substituting f = eE+ e - × B into…
A: Given :A charge particle in motion under the effect an electromagnetic field.Our task is to prove…
Q: e magnitude of the friction force (in Newton) wh
A:
Q: are using ropes atta the acceleration of th
A: 1. Toboggan in Deep Snow:Given:Mass of the toboggan (m) = 20 kgOpposing force (F_opposing) = 8 N…
Q: Method of Images: Two grounded conducting planes meet at right angles. In the region between them,…
A:
Q: collapse? if sa
A: High-density molecular clouds have stronger forces of gravity which push in and make it easier to…
Q: A 5-kg mass is attached to a spring with stiffness 135 N/m. The damping constant for the system is…
A:
Q: c) Figure 2 below shows hypothetical 3D unit cell of Orthorhombic Crystal Structure with lattice…
A: The objective of the question is to draw a reciprocal unit cell for the given Orthorhombic Crystal…
Q: ong wire is made of a metal with the same electron density as copper. The wire ed across the…
A: L=1.5mV=9v
Q: A laser pointer is placed on a platform that rotates at a rate of 19 revolutions per minute. The…
A:
Q: describe what modifications are needed in the general boundary conditions for electric field and…
A: The objective of the question is to understand the modifications required in the boundary conditions…
Q: proton and nucleus
A:
Q: e tension i
A: Given that:The system here is a plank attached to a wall with a hinge and a rope holding the plank…
Q: M x= -G +G M x2 (x — L)²' -
A: Given the equation d2xdt2=−GMx2+GM(x−L)2⇒d2xdt2=−GMx2+GM(L−x)2 [As (x-L)2=(L-x)2]This is a…

Step by step
Solved in 3 steps with 3 images
