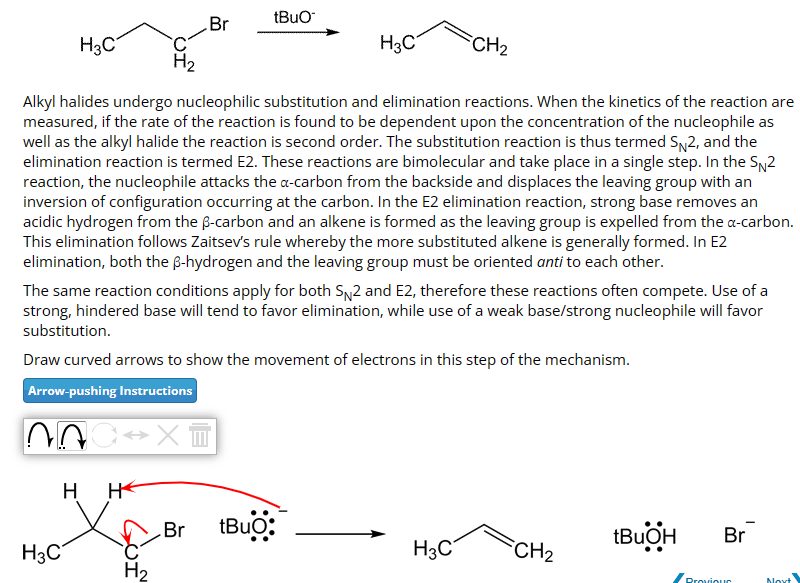
tBuO Br H3C H3C CH2 H2 Alkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent upon the concentration of the nucleophile as well as the alkyl halide the reaction is second order. The substitution reaction is thus termed SN2, and the elimination reaction is termed E2. These reactions are bimolecular and take place in a single step. In the SN2 reaction, the nucleophile attacks the a-carbon from the backside and displaces the leaving group with an inversion of configuration occurring at the carbon. In the E2 elimination reaction, strong base removes an acidic hydrogen from the ẞ-carbon and an alkene is formed as the leaving group is expelled from the a-carbon. This elimination follows Zaitsev's rule whereby the more substituted alkene is generally formed. In E2 elimination, both the ẞ-hydrogen and the leaving group must be oriented anti to each other. The same reaction conditions apply for both SN2 and E2, therefore these reactions often compete. Use of a strong, hindered base will tend to favor elimination, while use of a weak base/strong nucleophile will favor substitution. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions X H H Br tBuo: tBuOH Br H3C H3C CH2 H2 Proviour Noxt
Conditions which favor the SN1/E1 pathway include the use of a weak nucleophile and a polar protic solvent. The same reaction conditions apply for both SN1 and E1, therefore these reactions will compete. It is usually difficult to predict which pathway, substitution or elimination, will predominate and so a mixture of products is frequently observed.
In contrast to the E1 reaction which involves a carbocation intermediate, the E1cB reaction takes place through a carbanion intermediate. Base-induced abstraction of a proton in a slow, rate-limiting step gives an anion which then expels the leaving group from the adjacent carbon. The reaction is particularly suited to the elimination of substrates which contain poor leaving groups two carbons removed from a carbonyl group. The poor leaving group disfavors the alternative E1 and E2 reactions, and the carbonyl group helps to stabilize the anion via resonance.
Trending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images




