When 173. mg of a certain molecular compound X are dissolved in 100 g of cyclohexane (C6H12), the freezing point of the solution is measured to be 6.2 °C. Calculate the molar mass of X. If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. ロ・ロ x10
When 173. mg of a certain molecular compound X are dissolved in 100 g of cyclohexane (C6H12), the freezing point of the solution is measured to be 6.2 °C. Calculate the molar mass of X. If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. ロ・ロ x10
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 48QAP: The Rast method uses camphor (C10H16O) as a solvent for determining the molar mass of a compound....
Related questions
Question
Please help, i need fast answer.
don't provide handwritten solution ....
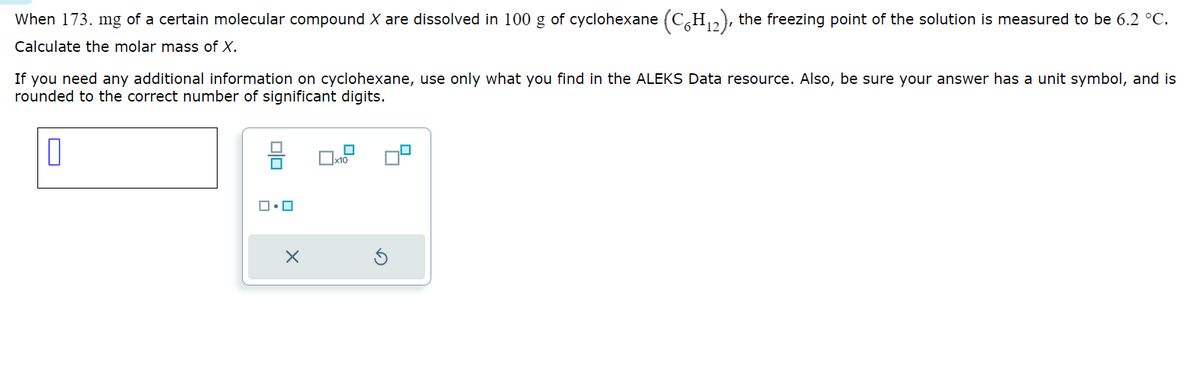
Transcribed Image Text:When 173. mg of a certain molecular compound X are dissolved in 100 g of cyclohexane (C6H12), the freezing point of the solution is measured to be 6.2 °C.
Calculate the molar mass of X.
If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is
rounded to the correct number of significant digits.
ロ・ロ
x10
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning