Sample bartleby Q&A Solution
You ask questions, our tutors answer
Browse
Question
A mixture of ideal gases has the following composition by mass :
Oxygen(60%), Carbon dioxide(20%),Nitrogen(20%)
Calculate the Characteristic gas constant of the mixture in J/kg-K.
Expert Answer
We can calculate the Characteristic gas constant for mixture of gasses by using the following formula.

Let Oxygen be gas1, Carbon dioxide be gas2 and Nitrogen be gas3.
Characteristic gas constant for gases 1,2 and 3 is calculated as follows
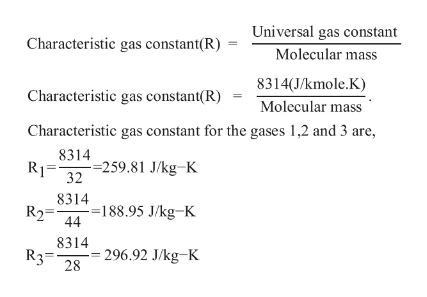
Substitute m1=0.6; m2=0.2kg; m3=0.2kg, R1=259.81 J/kg-K, R2=188.95 J/kg-K, R3=296.92 J/kg-K in Equation (1)

Characteristic gas constant of the mixture is 253.06 J/kg-K.