Sample bartleby Q&A Solution
You ask questions, our tutors answer
Browse
Atomic structureChemical ThermodynamicsEquilibrium ConceptsOrganic ChemistryPeriodicity in PropertiesRedox Reactions
Question
Determine the standard enthalpy of CH3OH from the following reactions.
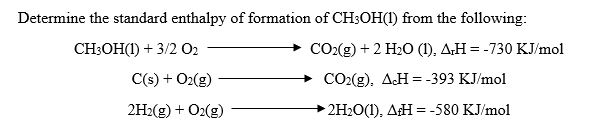
Transcribed Image Text
Determine the standard enthalpy of formation of CHsOH() from the following: CH3OH(I) + 3/2 02 CO2(g) + 2 H20 (I), Δ,Η =-730 KJ/mol C(s) O2(gCO2g) AcH-393 KJ/mol 2H2(g) + 02(g)2 2H201), AH 580 KJ/mol
Expert Answer
To calculate the standard enthalpy of formation of CH3OH(l), the required equation is,
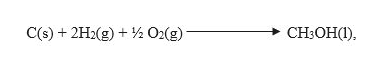
Now let us try to frame the required equation with the help of given equations,
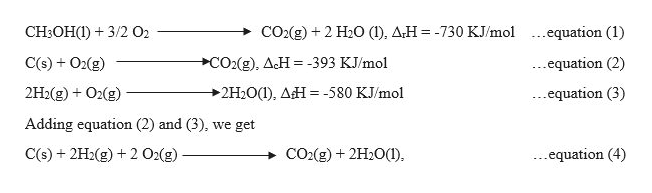
The enthalpy of formation for this reaction is calculated as,

Now since it is required to calculate the formation enthalpy of CH3OH(l), so reversing equation (1), and since the equation is reversed, therefore, the sign of ΔrH will also get changed and it becomes positive.
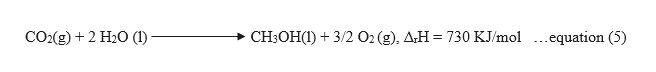
Now to obtain the required equation, let us add equation (4) and (5), we get,
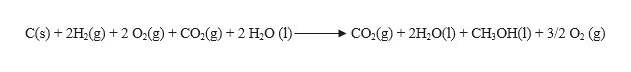
Smplifying the equation by removing the same terms on both the sides, we get the required equation,
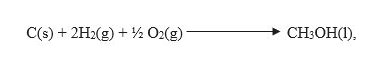
The standard enthalpy of formation of CH3OH is calculated as,
