Draw a Lewis structure for each of the following species:
- a. H2CO3
- b. CO32−
- c. CH2O
- d. CO2
(a)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
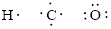
Hydrogen can form only one bond, carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
The Lewis structure can be,
 or
or 
Each atom has to achieve an octet and the existing electron has to be shared.
(b)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
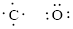
Carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
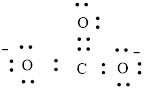 or
or 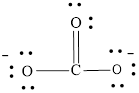
Each atom has to achieve an octet and the existing electron has to be shared.
(c)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
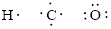
Hydrogen can form only one bond, carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
The Lewis structure can be,
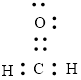 or
or 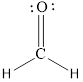
Each atom has to achieve an octet and the existing electron has to be shared.
(d)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,

Carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
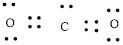 or
or 
Each atom has to achieve an octet and the existing electron has to be shared.