Interpretation:
Lone pair of electrons present on oxygen atom in the given structure has to be drawn.
Concept Introduction:
Lone pair of electrons are the pair of valence electrons that are not involved in bonding with other atoms. These are also called as non-bonding pair. Formal charge and lone pair of electrons are more important in representing a correct structure. If we know the formal charge on an atom, the lone pair of electrons can be found and vice-versa. The simple convention is to ignore the lone pairs and always show the formal charge. To find the lone pair of electrons present on an atom, few steps has to be followed and they are listed below,
- Valence electron of an atom has to be found according to the periodic table.
- Formal charge on the atom has to be considered. Positive charge means one electron less and a negative charge means one electron is more.
- The difference between the valence electron and the formal charge gives the lone pair of electrons that is present.
If the compound contains oxygen atom and there is no formal charge present on the oxygen atom means it will have two bonds and two lone pair of electrons.
If the oxygen atom has a formal positive charge means, then the oxygen atom will have three bonds and one lone pair of electrons.
If the oxygen atom has a formal negative charge means, then the oxygen atom will have one bond and three lone pair of electrons.
Answer
There are two lone pairs of electrons present on the oxygen atom in the given structure. It can be shown as,
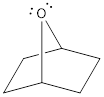
Explanation of Solution
Given structure is,
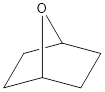
Oxygen is a group 6A element. The valence electron of oxygen is six. The oxygen atom in the given structure does not have any formal charge. The oxygen atom has two bonds and this means that out of six valence electrons, two are involved in bonding with other atoms. Therefore, the other four electrons must be two lone pair of electrons. Hence, oxygen atom in the given structure has two lone pair of electrons and it can be shown as,

Lone pair of electrons present on oxygen atom in the given structure was drawn.