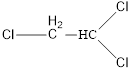(a)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
Explanation of Solution
Given molecular formula
Carbon is tetravalent and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,
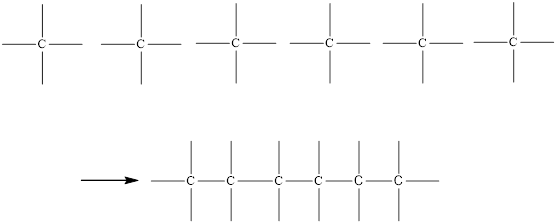
The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
On placing the hydrogen atom at the periphery, the isomers will be
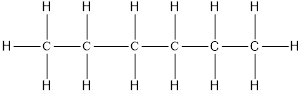
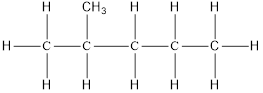
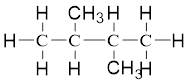
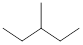 and
and 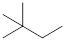
(b)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed only at positions, i.e. connected to any one of the carbon.
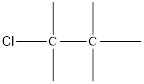
On placing the hydrogen atom at the periphery, the isomer will be
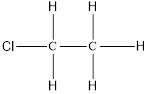
(c)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed at two positions,
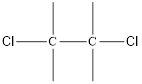 and
and 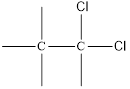
On placing the hydrogen atom at the periphery, the isomer will be
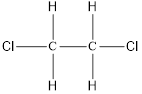 and
and 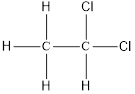
(c)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed at two positions.
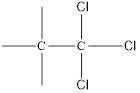 and
and 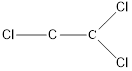
On placing the hydrogen atom at the periphery, the isomer will be
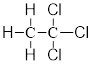 and
and