Interpretation:
The values of
Concept Introduction:
The mathematical expression which gives the relation between velocity of light, frequency and wavelength is:
Where,
The mathematical expression for wave number is expressed as:
Where,
The mathematical expression for energy is expressed as:
Where,
Answer
In figure 1.19, the values of wavelength and frequency are close to the calculated values.
Explanation of Solution
Given information:
The frequency is calculated as:
Convert the given value of wavelength in nm to m.
Since,
Thus,
Wavelength in m =
=
Put the values,
Wave number is calculated as:
Convert the given value of wavelength in nm to cm.
Since,
Thus,
Wavelength in m =
=
Put the values,
Energy is calculated as:
Put the values,
Figure 1.19 is shown as:
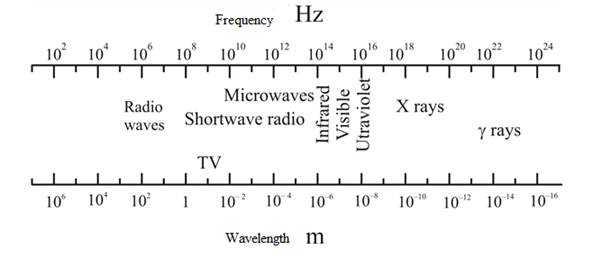
In the above figure, the value of wavelength and frequency is