1) Balance all these equations. (Hint, only small numbers are needed) 2) Determine the theoretical yield of the final solid if you use 20.000g NaHCO3 Possible equations for the decomposition. Mass of just Theoretical the NaHCO, yield of final solid A NAHCO () → _NazCO,•H20 6) + CO2) - B _NAHCO, (4) → NazCO () +_H2O + - C NaHCO3 () > NAOH )+ CO2 - - NaHCO () > _NazO () + H20 +- CO2o -
1) Balance all these equations. (Hint, only small numbers are needed) 2) Determine the theoretical yield of the final solid if you use 20.000g NaHCO3 Possible equations for the decomposition. Mass of just Theoretical the NaHCO, yield of final solid A NAHCO () → _NazCO,•H20 6) + CO2) - B _NAHCO, (4) → NazCO () +_H2O + - C NaHCO3 () > NAOH )+ CO2 - - NaHCO () > _NazO () + H20 +- CO2o -
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
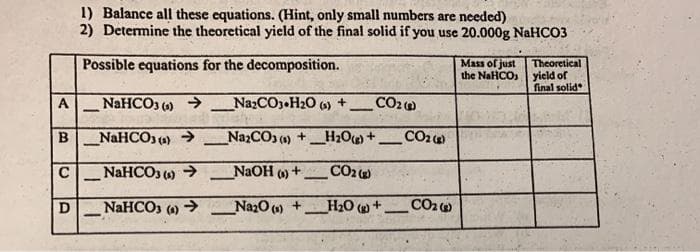
Transcribed Image Text:1) Balance all these equations. (Hint, only small numbers are nceded)
2) Determine the theoretical yield of the final solid if you use 20.000g NaHCO3
Possible equations for the decomposition.
Mass of just
the NaHCO
Theoretical
yicld of
final solid
A
NAHCO3 () >
NazCO3 H20 (6)
CO2
-
-
_NaHCO, (a) →
NazCO () +_H20 +_CO2
B
-
C
- NAHCO3 ) >
CO2
NaOH
(0)
-
NaHCO3
(4)
->
Na20 (0) +
H20 +.
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning