1) Energy dispersive x-ray spectroscopy (EDX) is a commonly used characterization technique in Materials Science to determine the elemental composition of materials. To do this, EDX measures the emitted characteristic x-rays from materials (e.g. Ka), generated due to the excitation of electrons from a lower energy shell to a higher energy one. Assume that you are working as a Materials Engineer in a company that produces cars. Your company has purchased 1 ton of Cr-Fe-Cu alloy to fabricate a certain part of the car. Your boss asks you to determine whether the material your company purchased is, in fact Cr-Fe-Cu alloy. If it there is another element present in the material, you will return the purchased material and stop production. So, you perform EDX analysis on your material and you see that three x-ray energies are detected: 5416, 6406 and 8050 eV. Determine which elements are present in your material and decide whether you should send the purchased material back or continue production. Wavelength of Ka x-rays Element | (nm) Cr 0,2291 Mn 0,2103 Fe 0,1937 Co 0,179 Ni 0,1659 Cu 0,1542 Zn 0,1436
1) Energy dispersive x-ray spectroscopy (EDX) is a commonly used characterization technique in Materials Science to determine the elemental composition of materials. To do this, EDX measures the emitted characteristic x-rays from materials (e.g. Ka), generated due to the excitation of electrons from a lower energy shell to a higher energy one. Assume that you are working as a Materials Engineer in a company that produces cars. Your company has purchased 1 ton of Cr-Fe-Cu alloy to fabricate a certain part of the car. Your boss asks you to determine whether the material your company purchased is, in fact Cr-Fe-Cu alloy. If it there is another element present in the material, you will return the purchased material and stop production. So, you perform EDX analysis on your material and you see that three x-ray energies are detected: 5416, 6406 and 8050 eV. Determine which elements are present in your material and decide whether you should send the purchased material back or continue production. Wavelength of Ka x-rays Element | (nm) Cr 0,2291 Mn 0,2103 Fe 0,1937 Co 0,179 Ni 0,1659 Cu 0,1542 Zn 0,1436
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.103PAE: 6.103 Atomic absorption spectroscopy is based on the atomic spectra of the elements being studied....
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
100%
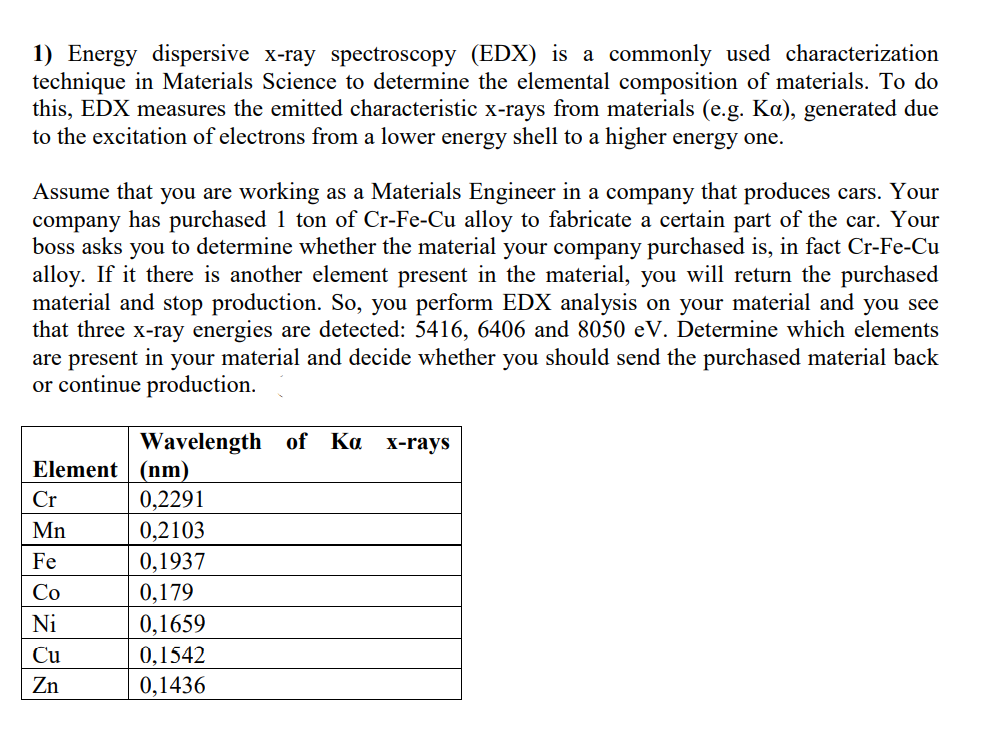
Transcribed Image Text:1) Energy dispersive x-ray spectroscopy (EDX) is a commonly used characterization
technique in Materials Science to determine the elemental composition of materials. To do
this, EDX measures the emitted characteristic x-rays from materials (e.g. Ka), generated due
to the excitation of electrons from a lower energy shell to a higher energy one.
Assume that you are working as a Materials Engineer in a company that produces cars. Your
company has purchased 1 ton of Cr-Fe-Cu alloy to fabricate a certain part of the car. Your
boss asks you to determine whether the material your company purchased is, in fact Cr-Fe-Cu
alloy. If it there is another element present in the material, you will return the purchased
material and stop production. So, you perform EDX analysis on your material and you see
that three x-ray energies are detected: 5416, 6406 and 8050 eV. Determine which elements
are present in your material and decide whether you should send the purchased material back
or continue production.
Wavelength of Ka
X-rays
Element (nm)
0,2291
0,2103
Cr
Mn
Fe
0,1937
Co
0,179
Ni
0,1659
Cu
0,1542
Zn
0,1436
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning