1 of 4 I Review | Constants | Periodic Tab Part A The goose has a mass of 24.8 lb (pounds) and is flying at 9.00 miles/h (miles per hour). What is the kinetic energy of the goose in joules? Enter your answer numerically in joules. View Available Hint(s) Π ΑΣΦ Submit Request Answer P Pearson nc. All rights reserved. | Terms of Use | Privacy Policy Permissions | Contact Us 1:05 PM 96% 2/26/202
1 of 4 I Review | Constants | Periodic Tab Part A The goose has a mass of 24.8 lb (pounds) and is flying at 9.00 miles/h (miles per hour). What is the kinetic energy of the goose in joules? Enter your answer numerically in joules. View Available Hint(s) Π ΑΣΦ Submit Request Answer P Pearson nc. All rights reserved. | Terms of Use | Privacy Policy Permissions | Contact Us 1:05 PM 96% 2/26/202
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.84E: Assume that 1.20 g of benzoicacid, C6H5COOH, is burned in a porcelain dish exposed to the air.If 31,...
Related questions
Question
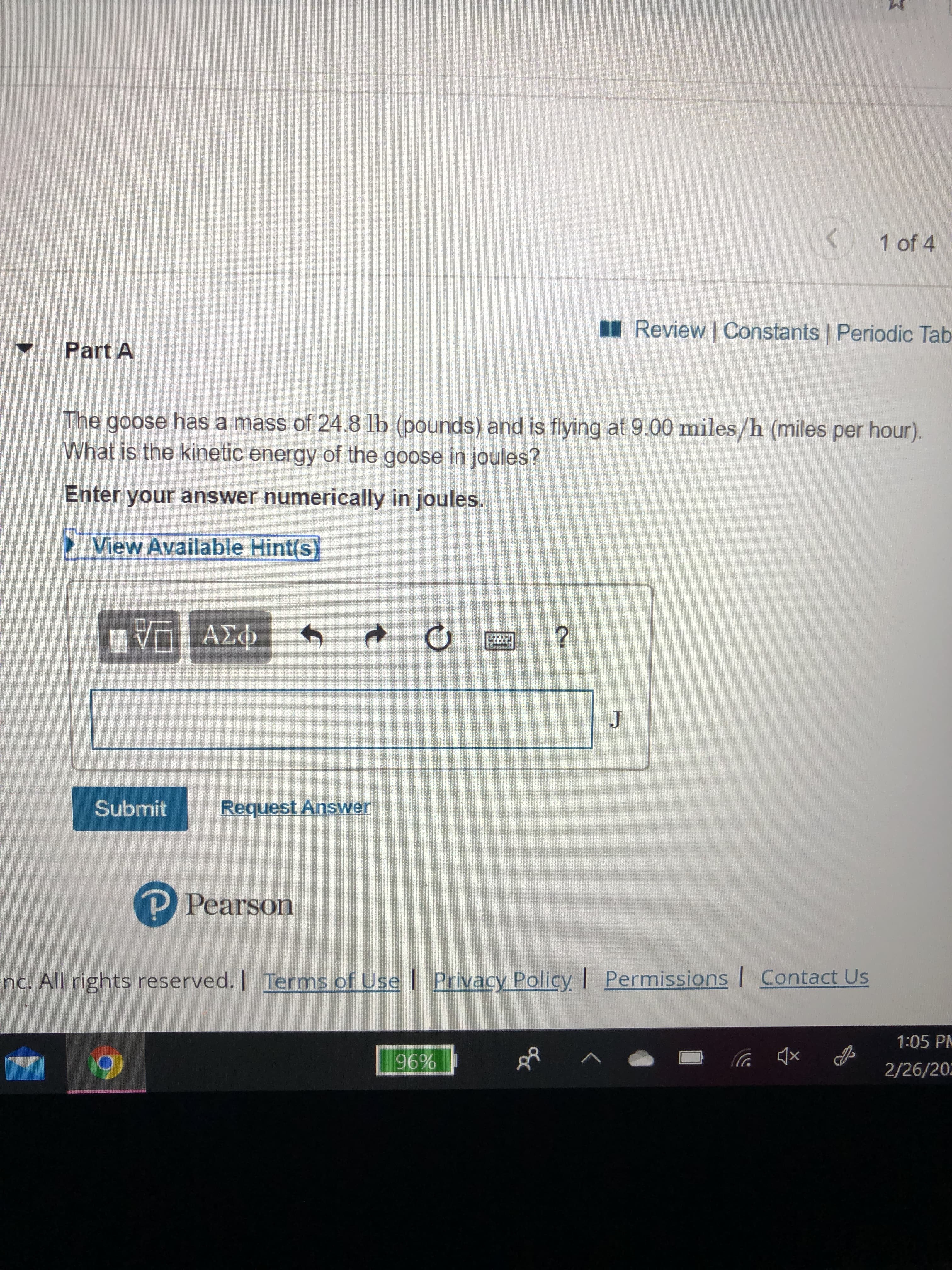
Transcribed Image Text:1 of 4
I Review | Constants | Periodic Tab
Part A
The goose has a mass of 24.8 lb (pounds) and is flying at 9.00 miles/h (miles per hour).
What is the kinetic energy of the goose in joules?
Enter your answer numerically in joules.
View Available Hint(s)
Π ΑΣΦ
Submit
Request Answer
P Pearson
nc. All rights reserved. | Terms of Use | Privacy Policy Permissions | Contact Us
1:05 PM
96%
2/26/202
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning