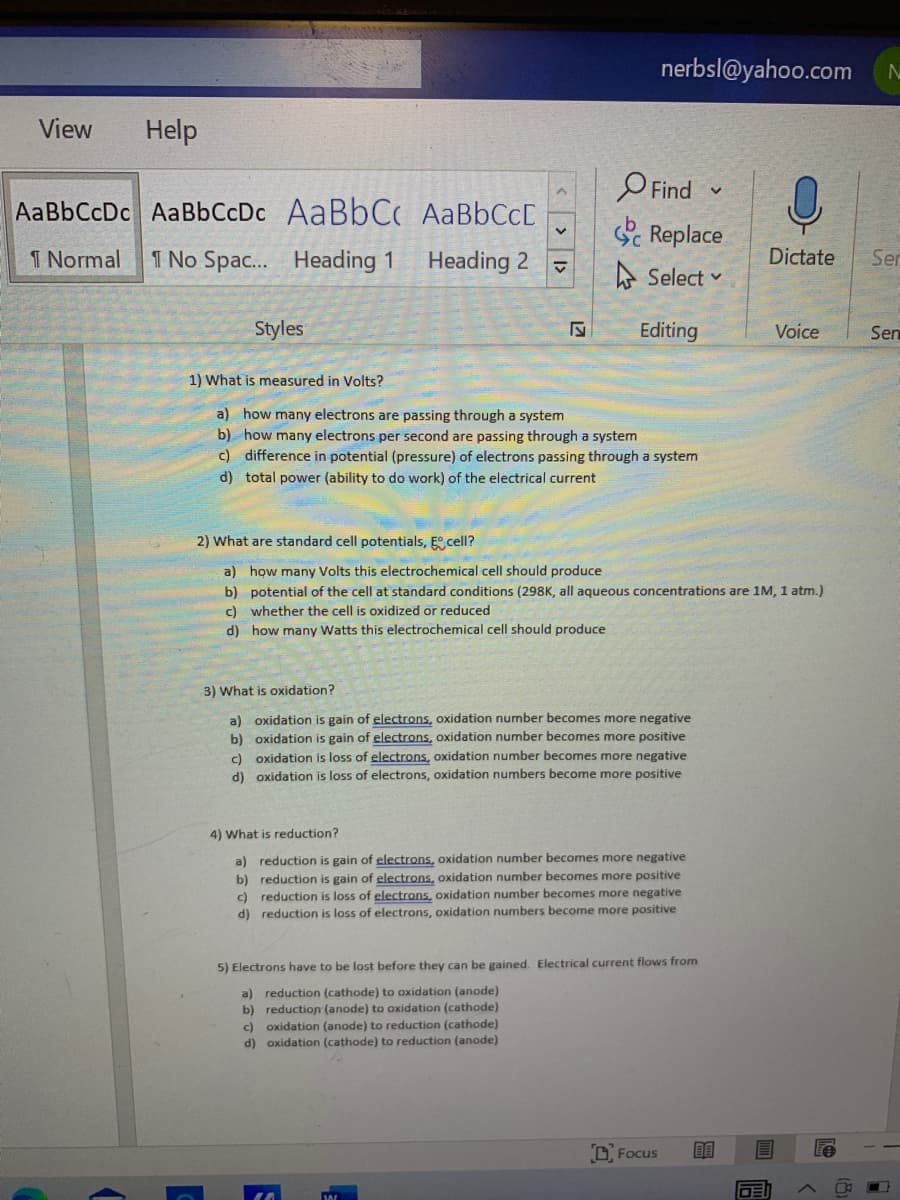
1) What is measured in Volts? a) how many electrons are passing through a system b) how many electrons per second are passing through a system c) difference in potential (pressure) of electrons passing through a system d) total power (ability to do work) of the electrical current 2) What are standard cell potentials, E cell? a) how many Volts this electrochemical cell should produce b) potential of the cell at standard conditions (298K, all aqueous concentrations are 1M, 1 atm.) c) whether the cell is oxidized or reduced d) how many Watts this electrochemical cell should produce 3) What is oxidation? a) oxidation is gain of electrons, oxidation number becomes more negative b) oxidation is gain of electrons, oxidation number becomes more positive c) oxidation is loss of electrons, oxidation number becomes more negative d) oxidation is loss of electrons, oxidation numbers become more positive
1) What is measured in Volts? a) how many electrons are passing through a system b) how many electrons per second are passing through a system c) difference in potential (pressure) of electrons passing through a system d) total power (ability to do work) of the electrical current 2) What are standard cell potentials, E cell? a) how many Volts this electrochemical cell should produce b) potential of the cell at standard conditions (298K, all aqueous concentrations are 1M, 1 atm.) c) whether the cell is oxidized or reduced d) how many Watts this electrochemical cell should produce 3) What is oxidation? a) oxidation is gain of electrons, oxidation number becomes more negative b) oxidation is gain of electrons, oxidation number becomes more positive c) oxidation is loss of electrons, oxidation number becomes more negative d) oxidation is loss of electrons, oxidation numbers become more positive
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.72PAE: 9.72 Although it can be a nuisance when a laptop computer freezes up and needs to be rebooted, we...
Related questions
Question
Transcribed Image Text:nerbsl@yahoo.com
View
Help
O Find
S Replace
AaBbCcDc AaBbCcDc AaBbCc AaBbCcD
I Normal
1 No Spac. Heading 1
Heading 2
Dictate
Ser
A Select v
Styles
Editing
Voice
Sen
1) What is measured in Volts?
a) how many electrons are passing through a system
b) how many electrons per second are passing through a system
c) difference in potential (pressure) of electrons passing through a system
d) total power (ability to do work) of the electrical current
2) What are standard cell potentials, E cell?
a) how many Volts this electrochemical cell should produce
b) potential of the cell at standard conditions (298K, all aqueous concentrations are 1M, 1 atm.)
c) whether the cell is oxidized or reduced
d) how many Watts this electrochemical cell should produce
3) What is oxidation?
a) oxidation is gain of electrons, oxidation number becomes more negative
b) oxidation is gain of electrons, oxidation number becomes more positive
c) oxidation is loss of electrons, oxidation number becomes more negative
d) oxidation is loss of electrons, oxidation numbers become more positive
4) What is reduction?
a) reduction is gain of electrons, oxidation number becomes more negative
b) reduction is gain of electrons, oxidation number becomes more positive
c) reduction is loss of electrons, oxidation number becomes more negative
d) reduction is loss of electrons, oxidation numbers become more positive
5) Electrons have to be lost before they can be gained. Electrical current flows from
a) reduction (cathode) to oxidation (anode)
b) reduction (anode) to oxidation (cathode)
c) oxidation (anode) to reduction (cathode)
d) oxidation (cathode) to reduction (anode)
D, Focus
OE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning