1. (6 points) For the equilibrium 2 IBr(g) g) +Brg), Kp 8.5x 10-3 at 150 °C. If 0.025 atm of IBr is placed in a 1.0-L container, what are the partial pressures of IBr, I2 and Br2 at equilibrium? . C1l e fLe nd 2 00o mal at 44 C. The
1. (6 points) For the equilibrium 2 IBr(g) g) +Brg), Kp 8.5x 10-3 at 150 °C. If 0.025 atm of IBr is placed in a 1.0-L container, what are the partial pressures of IBr, I2 and Br2 at equilibrium? . C1l e fLe nd 2 00o mal at 44 C. The
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 7E: Explain why an equilibrium between Br2(l) and Br2(g) would not be established if the container were...
Related questions
Question
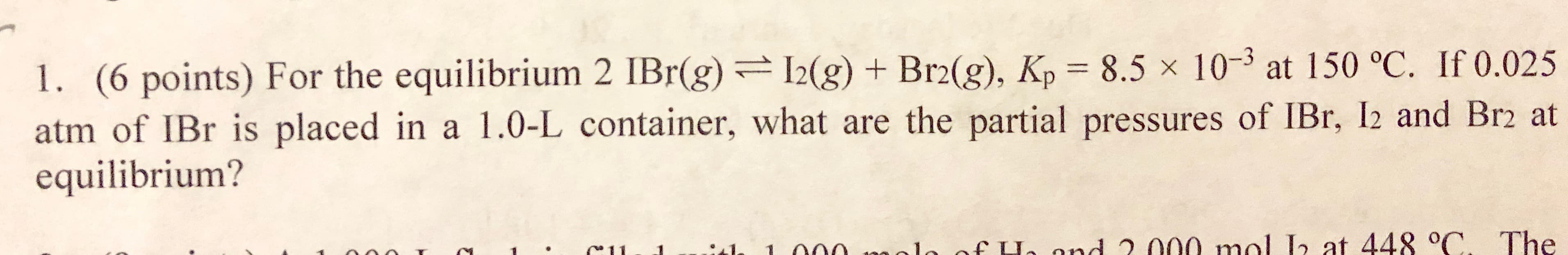
Transcribed Image Text:1. (6 points) For the equilibrium 2 IBr(g) g) +Brg), Kp 8.5x 10-3 at 150 °C. If 0.025
atm of IBr is placed in a 1.0-L container, what are the partial pressures of IBr, I2 and Br2 at
equilibrium?
. C1l e fLe nd 2 00o mal
at 44 C. The
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning