1. a. Estimate the % uncertainty in your measurement of: mass of water mass of metal AT of water Which % uncertainty is greatest? Which measurement is most critical? b. For your calculated specific heat, determine: (1) % uncertainty AT of metal (2) absolute uncertainty (3) how many significant figures your reported value should have.
1. a. Estimate the % uncertainty in your measurement of: mass of water mass of metal AT of water Which % uncertainty is greatest? Which measurement is most critical? b. For your calculated specific heat, determine: (1) % uncertainty AT of metal (2) absolute uncertainty (3) how many significant figures your reported value should have.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.26E
Related questions
Question
Please answer all questions and show calculations
For the image attached
For 1. a
Mass of metal:
Trial 1 is 35.0228 g
Trial 2 is 35.0915 g
Trial 3 is 34.0821 g
Mass of water:
Trial 1 is 20.0177 g
Trial 2 is 20.0250 g
Trial 3 is 20.0168 g
For delta t of water:
Trial 1 is 15.5 C
Trial 2 is 15.7 C
Trial 3 is 15.1 C
For delta t of metal
Trial 1 is 80.1 C
Trial 2: 80.2 C
Trial 3: 79.5 C
For B my calculated Specific heat is:
Trial 1 is 0.462
Trial 2 is 0.467
Trial 3 is 0.466
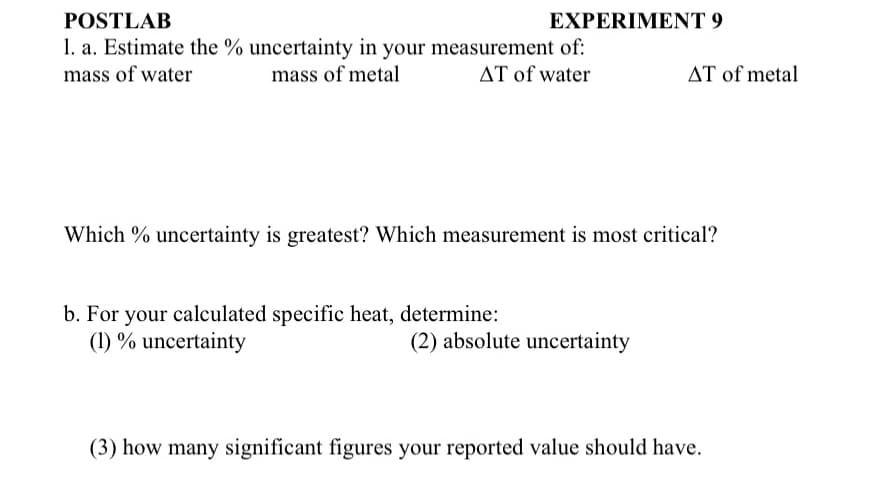
Transcribed Image Text:POSTLAB
1. a. Estimate the % uncertainty in your measurement of:
mass of water
mass of metal
AT of water
EXPERIMENT 9
b. For your calculated specific heat, determine:
(1) % uncertainty
Which % uncertainty is greatest? Which measurement is most critical?
AT of metal
(2) absolute uncertainty
(3) how many significant figures your reported value should have.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning