1. Balance the following redox reactions. Show your work. Mn2+ (in acidic solution) I a. Mn0 I2 b. SO3 Mn04SO2Mn02 (in basic solution) Fe02 CI (in acidic solution) C. Fe(OH)3 + OcI - d. VO3 Fe2+ -> VO2+ + Fe3+ (in acidic solution)
1. Balance the following redox reactions. Show your work. Mn2+ (in acidic solution) I a. Mn0 I2 b. SO3 Mn04SO2Mn02 (in basic solution) Fe02 CI (in acidic solution) C. Fe(OH)3 + OcI - d. VO3 Fe2+ -> VO2+ + Fe3+ (in acidic solution)
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 19Q: What is electrochemistry? What are redox reactions? Explain the difference between a galvanic and an...
Related questions
Question
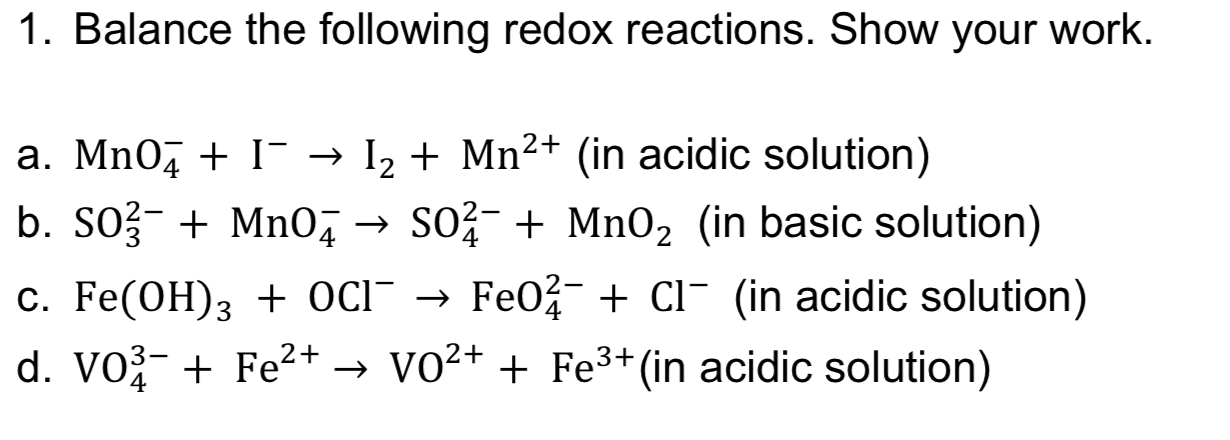
Transcribed Image Text:1. Balance the following redox reactions. Show your work.
Mn2+ (in acidic solution)
I
a. Mn0
I2
b. SO3 Mn04SO2Mn02 (in basic solution)
Fe02 CI (in acidic solution)
C. Fe(OH)3 + OcI -
d. VO3 Fe2+ -> VO2+ + Fe3+ (in acidic solution)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 6 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning