1. Complete th e preparations of some transuranium elements (elements with atomic numbers greater th an 92, Classify also the reaction as (a) radioactive decay (b) nuclear transmutation, (c) nuclear fusion, (d) nuclear fission. 23U + ¿n → 233Np a. b. 233NP + -{ß 렁릉Cm + 24 Cf + ¿n C. d. 208Pb + + 공n 23Cf + + 4 in e. 2. Fill in the blanks in the foilowing radioactive decay series: 232TH 2, 228Th a. S 227 Ac b. 235U 3 4 233Pa £ C. 3. Write balanced nuclear equations for the followwing reactions and identiry X: a. X (p,a) 1¿c 3Se (d.p) x b. ?{Al (d,a) X e. X (d,2p) ¿Li c. Mn (n.Y) x f. 1gB (n,a) X d. 4. Write the balanced nuclear equations for the following: Beta decay of s odium-26 Alpha decay of 21293 Bi Electron capture by 11049ln Alpha decay of polonium-218 Production of ²4'95 Am through beta decay Formation of 20383 Bi through alpha decay Formation of 52 Formation of Polonium-215 through alpha decay Production of ²2®aAc through beta decay Formation of 81Kr through electron capture a. b. C. d. e. f. Mn through positron emission g. h. i. j.
1. Complete th e preparations of some transuranium elements (elements with atomic numbers greater th an 92, Classify also the reaction as (a) radioactive decay (b) nuclear transmutation, (c) nuclear fusion, (d) nuclear fission. 23U + ¿n → 233Np a. b. 233NP + -{ß 렁릉Cm + 24 Cf + ¿n C. d. 208Pb + + 공n 23Cf + + 4 in e. 2. Fill in the blanks in the foilowing radioactive decay series: 232TH 2, 228Th a. S 227 Ac b. 235U 3 4 233Pa £ C. 3. Write balanced nuclear equations for the followwing reactions and identiry X: a. X (p,a) 1¿c 3Se (d.p) x b. ?{Al (d,a) X e. X (d,2p) ¿Li c. Mn (n.Y) x f. 1gB (n,a) X d. 4. Write the balanced nuclear equations for the following: Beta decay of s odium-26 Alpha decay of 21293 Bi Electron capture by 11049ln Alpha decay of polonium-218 Production of ²4'95 Am through beta decay Formation of 20383 Bi through alpha decay Formation of 52 Formation of Polonium-215 through alpha decay Production of ²2®aAc through beta decay Formation of 81Kr through electron capture a. b. C. d. e. f. Mn through positron emission g. h. i. j.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
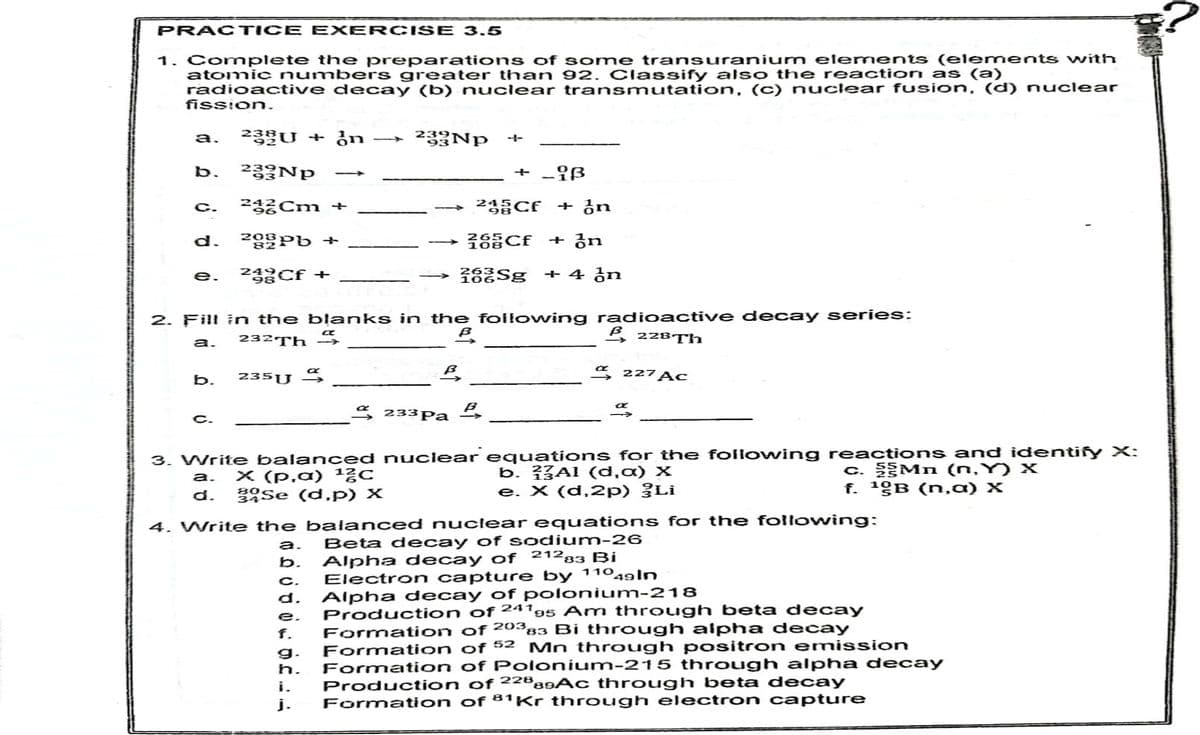
Transcribed Image Text:PRACTICE EXERCIS E 3.5
1. Complete the preparations of some transuranium elements (elements with
atomic numbers greater than 92. Classify also the reaction as (a)
radioactive decay (b) nuclear transmutation, (c) nuclear fusion, (d) nuclear
fission.
23U + ¿n
» 239NP
a.
233Np
+ -Iß
b.
렁릉Cm +
23 CE + on
C.
d.
로등들CF + an
23Cf +
+ 4 ¿n
e.
2. Fill in the blanks in the foll owing radioactive decay series:
B, 228Th
а.
232Th
a 227 AC
b.
235U 3
e 233Pa
C.
3. Write balanced nuclear equations for the foilowing reactions and identify X:
X (p,a) 1층C
Se (d,p) X
b. 3Al (d,a) X
e. X (d,2p) ¿Li
c. Mn (n, Y) X
f. 1gB (n,a) X
а.
d.
4. Write the balanced nuclear equations for the following:
Beta decay of sodium-26
Alpha decay of 21283 Bi
Electron capture by 11049ln
Alpha decay of polonium-218
Production of 24195 Am through beta decay
Formation of 20383 Bi through alpha decay
Formation of 52 Mn through positron emission
Formation of Polonium-215 through alpha decay
Production of ²2®89AC through beta decay
Formation of 81Kr through electron capture
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
--cס
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY