1. Consider the following reaction: 2 NO2 2 NO 02 At equilibrium, there are 0.035 mol of NO2, 0.045 mol of NO2 and 0.023 mol of O2 are in the 1.07L reaction flask. Calculate the Keq for this reaction. 3. Consider the reaction 2HI H2(g) H2 g) (g) The partial pressures of H2 and I2 at equilibrium are 0.710 and 0.888atm, respectively What is the equilibrium partial pressure of HI? Given Keq = 0.324
1. Consider the following reaction: 2 NO2 2 NO 02 At equilibrium, there are 0.035 mol of NO2, 0.045 mol of NO2 and 0.023 mol of O2 are in the 1.07L reaction flask. Calculate the Keq for this reaction. 3. Consider the reaction 2HI H2(g) H2 g) (g) The partial pressures of H2 and I2 at equilibrium are 0.710 and 0.888atm, respectively What is the equilibrium partial pressure of HI? Given Keq = 0.324
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 30QAP: . Consider the reaction 2CO(g)+O2(g)2CO2(g)Suppose the system is already at equilibrium, and then an...
Related questions
Question
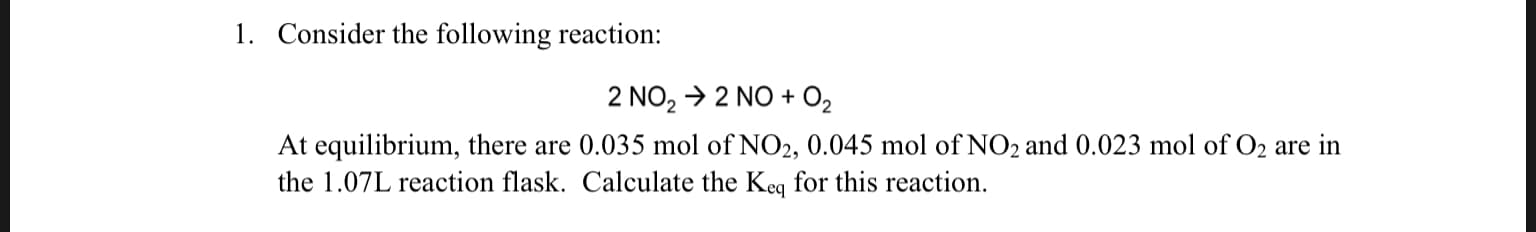
Transcribed Image Text:1. Consider the following reaction:
2 NO2 2 NO 02
At equilibrium, there are 0.035 mol of NO2, 0.045 mol of NO2 and 0.023 mol of O2 are in
the 1.07L reaction flask. Calculate the Keq for this reaction.

Transcribed Image Text:3. Consider the reaction 2HI
H2(g)
H2 g)
(g)
The partial pressures of H2 and I2 at equilibrium are 0.710 and 0.888atm, respectively
What is the equilibrium partial pressure of HI? Given Keq = 0.324
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning