1. Suppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass 58.0 grams 50.1 grams Volume Stone 1 Stone 2 20.0 cm3 15.0 cm3
1. Suppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass 58.0 grams 50.1 grams Volume Stone 1 Stone 2 20.0 cm3 15.0 cm3
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 95QRT
Related questions
Question
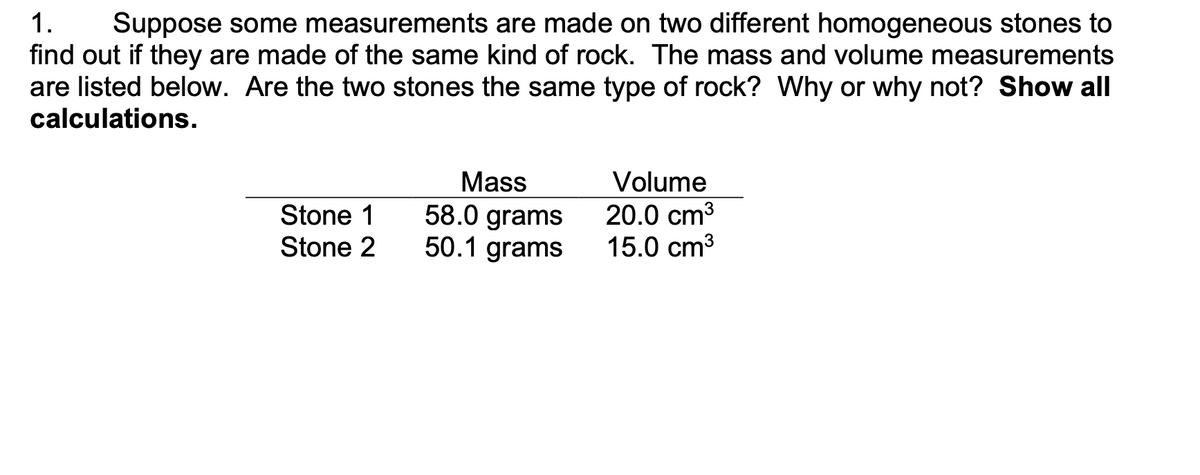
Transcribed Image Text:1.
Suppose some measurements are made on two different homogeneous stones to
find out if they are made of the same kind of rock. The mass and volume measurements
are listed below. Are the two stones the same type of rock? Why or why not? Show all
calculations.
Mass
Volume
58.0 grams
50.1 grams
20.0 cm3
15.0 cm3
Stone 1
Stone 2
Expert Solution
Step 1
Mass and volume are extensive properties which depends upon the amount of the substance and thus, cannot be used to predict the nature of the substance. Whereas mass and volume can be used to calculate density of a substance which is an intensive property and remains constant for a particular substance irrespective of the amount of the substance.
Density is calculated as the mass divided by the volume of the substance.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax