( 10 Maacular eauation Predict products, writing correct formula for each reactant and product, then provide coefficients to balance. Use chemical formula subscripts to denote state of each reactant and product. Assume all reactants are in aqueous solution. Coacg)+Ha0 ce) 3. 4. For reactions given in (3) write complete ionic equation and net ionic equation. 20 1+ (a) Na Cos sodium carbonate + hydrochloric acid Nt Hat HCL+ COs (a) Og(9t Na cot Hal (OH potassium hydroxide + hydrobromic acid H20 (L) Naelegt COat HaD (e) nt BR (b) (b) Ht br H OH nOH t HBr hbr t H20 (c) (aq) sodium chloride + sodium carbonate (04) aB (c) (d) Pb + No lead (II) nitrate + sodium chloride - NoT (d) Shift 2+ PbNDNa 2- Nn NOt PhCa SOy ba mourular equatian Complet Tauc (e) egbahan Net lonuc equotion- S0a tba (aa) PBas0 () 2+ (e) sodium sulfate + barium chloride Ctri > (f) (n) sodium carbonate + calcium chloride (g) (g) potassium hydroxide + magnesium nitrate - MgNala +1 ) Naag+SotClND a ANaatNc NR KHO + (h) sodium sulfate + copper (II) nitrate -1 +2 2 Nasit CuNos NoCuSe) (i) Ca) hydrochloric acid + potassium hydrogen carbonate (i) strontium nitrate + potassium phosphate -> Sr NDdst hapay
( 10 Maacular eauation Predict products, writing correct formula for each reactant and product, then provide coefficients to balance. Use chemical formula subscripts to denote state of each reactant and product. Assume all reactants are in aqueous solution. Coacg)+Ha0 ce) 3. 4. For reactions given in (3) write complete ionic equation and net ionic equation. 20 1+ (a) Na Cos sodium carbonate + hydrochloric acid Nt Hat HCL+ COs (a) Og(9t Na cot Hal (OH potassium hydroxide + hydrobromic acid H20 (L) Naelegt COat HaD (e) nt BR (b) (b) Ht br H OH nOH t HBr hbr t H20 (c) (aq) sodium chloride + sodium carbonate (04) aB (c) (d) Pb + No lead (II) nitrate + sodium chloride - NoT (d) Shift 2+ PbNDNa 2- Nn NOt PhCa SOy ba mourular equatian Complet Tauc (e) egbahan Net lonuc equotion- S0a tba (aa) PBas0 () 2+ (e) sodium sulfate + barium chloride Ctri > (f) (n) sodium carbonate + calcium chloride (g) (g) potassium hydroxide + magnesium nitrate - MgNala +1 ) Naag+SotClND a ANaatNc NR KHO + (h) sodium sulfate + copper (II) nitrate -1 +2 2 Nasit CuNos NoCuSe) (i) Ca) hydrochloric acid + potassium hydrogen carbonate (i) strontium nitrate + potassium phosphate -> Sr NDdst hapay
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
Not understanding the balancing of formulas or the the complete ionic and net ionic equations.
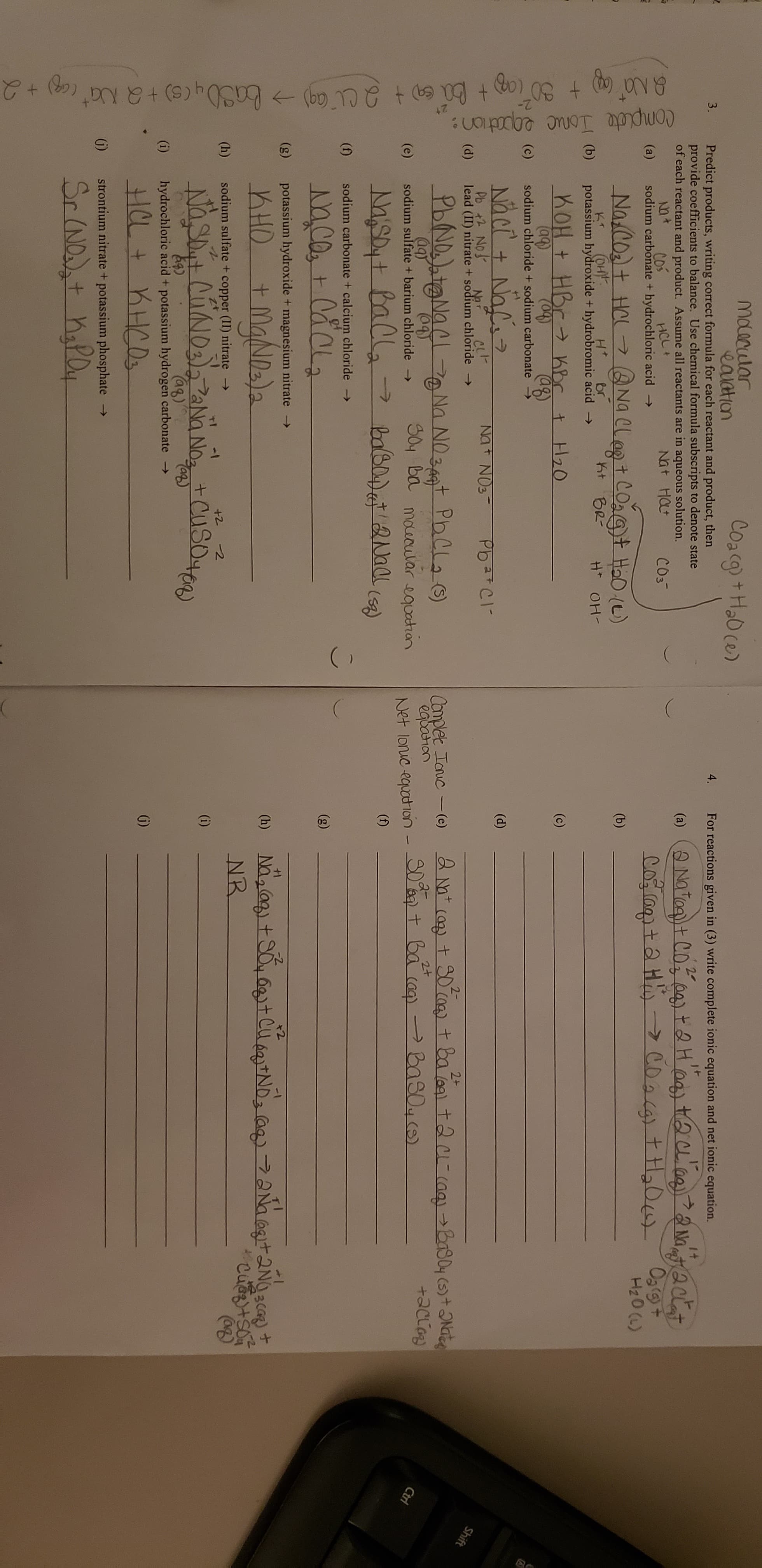
Transcribed Image Text:(
10
Maacular
eauation
Predict products, writing correct formula for each reactant and product, then
provide coefficients to balance. Use chemical formula subscripts to denote state
of each reactant and product. Assume all reactants are in aqueous solution.
Coacg)+Ha0 ce)
3.
4.
For reactions given in (3) write complete ionic equation and net ionic equation.
20
1+
(a)
Na
Cos
sodium carbonate + hydrochloric acid
Nt Hat
HCL+
COs
(a)
Og(9t
Na cot Hal
(OH
potassium hydroxide + hydrobromic acid
H20 (L)
Naelegt COat HaD (e)
nt BR
(b)
(b)
Ht
br
H OH
nOH t HBr hbr t H20
(c)
(aq)
sodium chloride + sodium carbonate
(04)
aB
(c)
(d)
Pb + No
lead (II) nitrate + sodium chloride -
NoT
(d)
Shift
2+
PbNDNa
2-
Nn NOt PhCa
SOy ba mourular equatian
Complet Tauc (e)
egbahan
Net lonuc equotion- S0a tba (aa) PBas0 ()
2+
(e)
sodium sulfate + barium chloride
Ctri
>
(f)
(n)
sodium carbonate + calcium chloride
(g)
(g)
potassium hydroxide + magnesium nitrate -
MgNala
+1
) Naag+SotClND a ANaatNc
NR
KHO
+
(h)
sodium sulfate + copper (II) nitrate
-1
+2 2
Nasit CuNos NoCuSe)
(i)
Ca)
hydrochloric acid + potassium hydrogen carbonate
(i)
strontium nitrate + potassium phosphate
->
Sr NDdst hapay
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co