10 of 12 + Solution Stoichiometry I Review | Constants | Periodic Table The stoichiometric relationship between the reactants and products of an aqueous reaction can be used to determine different kinds of information about a reaction, such as the volume of a given molarity reactant required to produce a certain amount of product. Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCI(aq) + Na2CO3 (aq)→2N2CI(aq) + H2O(1)+ CO2(g) Part A What volume of 2.50 MHC1 in liters is needed to react completely (with nothing left over) with 0.250 L of 0.100 M Na2CO3? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA Previous Answers Submit X Incorrect; Try Again; 10 attempts remaining
10 of 12 + Solution Stoichiometry I Review | Constants | Periodic Table The stoichiometric relationship between the reactants and products of an aqueous reaction can be used to determine different kinds of information about a reaction, such as the volume of a given molarity reactant required to produce a certain amount of product. Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCI(aq) + Na2CO3 (aq)→2N2CI(aq) + H2O(1)+ CO2(g) Part A What volume of 2.50 MHC1 in liters is needed to react completely (with nothing left over) with 0.250 L of 0.100 M Na2CO3? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA Previous Answers Submit X Incorrect; Try Again; 10 attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
100%
What volume of 2.50 MM HClHCl in liters is needed to react completely (with nothing left over) with 0.250 LL of 0.100 MM Na2CO3Na2CO3?
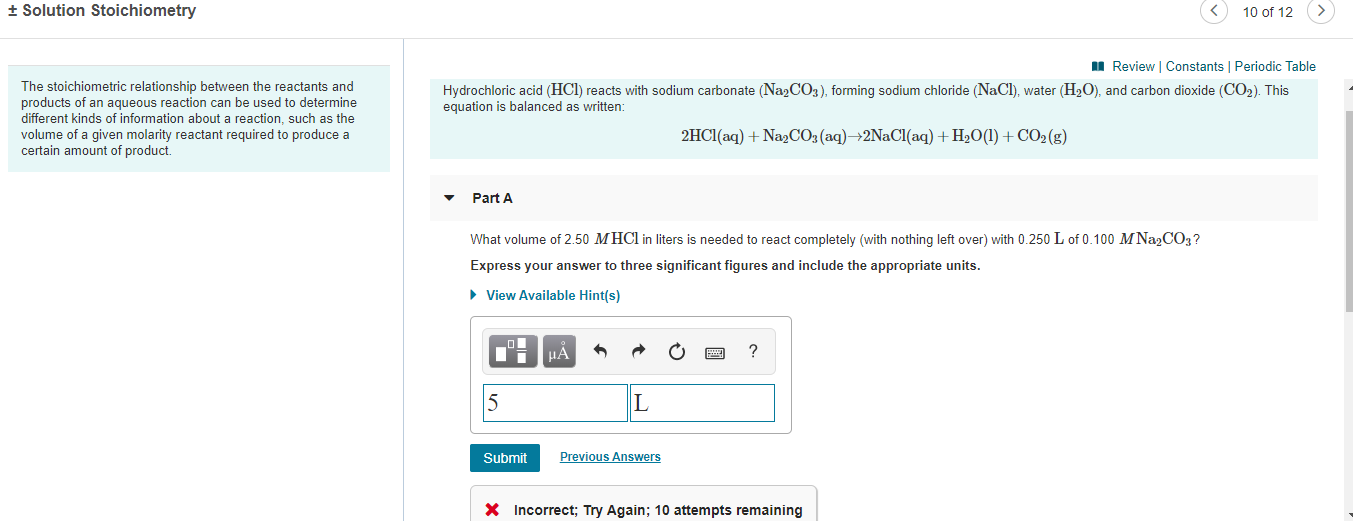
Transcribed Image Text:10 of 12
+ Solution Stoichiometry
I Review | Constants | Periodic Table
The stoichiometric relationship between the reactants and
products of an aqueous reaction can be used to determine
different kinds of information about a reaction, such as the
volume of a given molarity reactant required to produce a
certain amount of product.
Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This
equation is balanced as written:
2HCI(aq) + Na2CO3 (aq)→2N2CI(aq) + H2O(1)+ CO2(g)
Part A
What volume of 2.50 MHC1 in liters is needed to react completely (with nothing left over) with 0.250 L of 0.100 M Na2CO3?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
HA
Previous Answers
Submit
X Incorrect;
Try Again; 10 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning