10. Natural rubidium is composed of the isotopes 8Rb, which has an actual mass of 84.9117 amu, and °'Rb. Given that the abundance ratio of 8Rb/Rb in natural rubidium is 2.591, calculate the actual mass of rubidium-87 ( Rb). 87
10. Natural rubidium is composed of the isotopes 8Rb, which has an actual mass of 84.9117 amu, and °'Rb. Given that the abundance ratio of 8Rb/Rb in natural rubidium is 2.591, calculate the actual mass of rubidium-87 ( Rb). 87
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.48PAE: A materials engineer has filed for a patent for a new alloy to be used in golf club heads. The...
Related questions
Question
How do we solve this step by step?
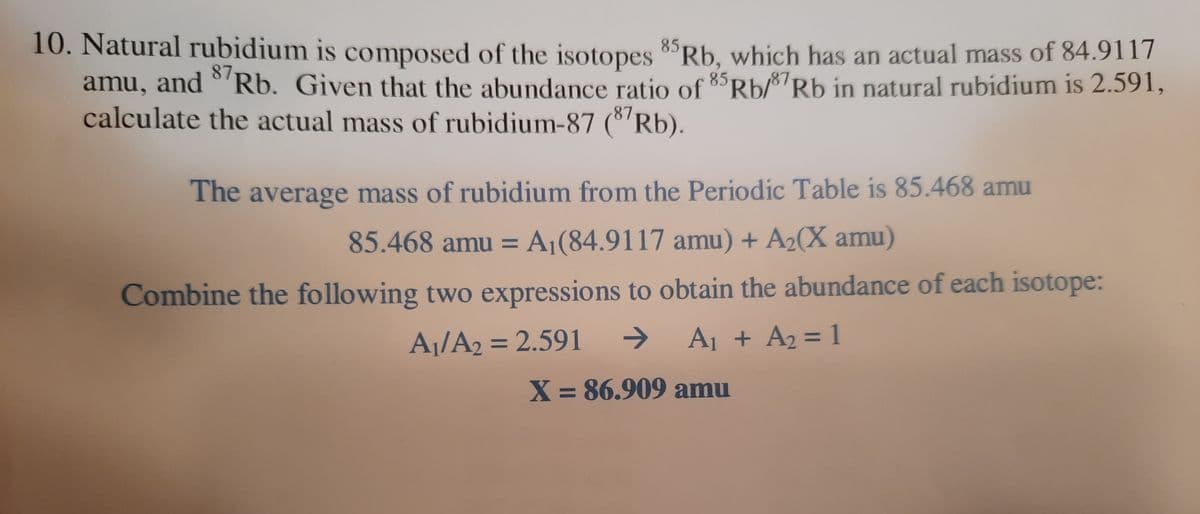
Transcribed Image Text:10. Natural rubidium is composed of the isotopes 8Rb, which has an actual mass of 84.9117
amu, and °'Rb. Given that the abundance ratio of 85Rb/87Rb in natural rubidium is 2.591,
calculate the actual mass of rubidium-87 (8'Rb).
87
The average mass of rubidium from the Periodic Table is 85.468 amu
85.468 amu = A¡(84.9117 amu) + A2(X amu)
Combine the following two expressions to obtain the abundance of each isotope:
A¡/A2 = 2.591
->
A1 + A2 = 1
%3D
X = 86.909 amu
%3D
Expert Solution
Step 1
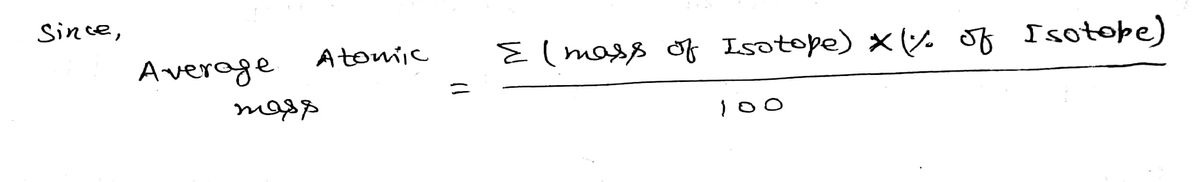
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning