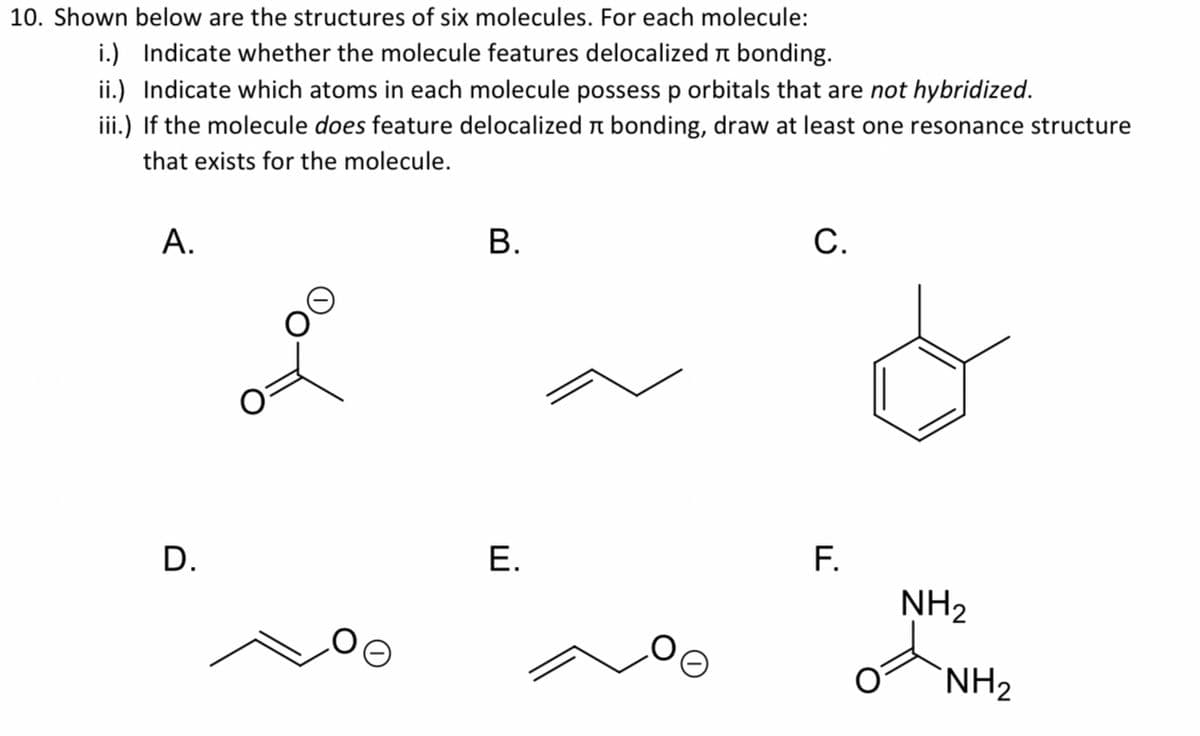
10. Shown below are the structures of six molecules. For each molecule: i.) Indicate whether the molecule features delocalized n bonding. ii.) Indicate which atoms in each molecule possess p orbitals that are not hybridized. iii.) If the molecule does feature delocalized n bonding, draw at least one resonance structure that exists for the molecule. А. В. С. F. NH2 D. Е. `NH2
Q: What are the angles a and b in the actual molecule of which this is a Lewis structure? :0: H- --…
A: Lewis acid structure of formic acid and it's different bond angel.
Q: 1. Neatly draw the Lewis dot structure. (Must be hand-drawn and very neat) 2. Count and report the…
A: “Since you have asked multiple question, we will solve the first question (first molecule: H2O) for…
Q: Azide (N3-) is a linear molecule. Analyze the structure and bonding of azide by preparing molecular…
A:
Q: question is related to valence bond theory. . Explain the assumptions made in bonding models under…
A: Since your question has multiple sub-parts, we will answer the first three sub-parts for you. If you…
Q: Please fill in the blank ! In part B, the difference is that the molecules have more than one atom…
A: VSEPR theory explain the molecular geometry based on electron pair present around the central atom.…
Q: C. Total atomic orbitals for both neon atoms? d. How many molecular orbitals would the two neon…
A: Since you have questions with the multiple subparts we will solve the first 3 subparts for you. If…
Q: Why is it important that the sp2 hybrid orbitals of the twocarbon atoms lie in the same plane?
A: The formation of sp2 hybrid orbital takes place when one s and two p orbitals of the same shell of…
Q: A newly discovered element Rm has 6 valence electrons. How many total valence electrons are in the…
A: Valence electron: The total number of electrons present in the outermost shell or valence shell is…
Q: Consider the structure of nicotine below and answer the questions that follow. nicotine a) What is…
A:
Q: Examine the model structures for NH3and CH4. Both these structures have the same electron geometry…
A:
Q: i.) Indicate whether the molecule features delocalized n bonding. ii.) Indicate which atoms in each…
A: i) delocalized pi bonding: two double bonds are adjacent or one double bond is attached to hetero…
Q: Consider the molecule NCS-1. Draw the Lewis dot diagram for this molecular ion, including resonance…
A: In NCS-, the centre atom is least electronegative carbon. Since there are 5 valence electrons in N.…
Q: Use valence bond theory to describe the hybridized bonding in the following molecule. (i) What is…
A:
Q: The structure of caffeine is shown below. (a) Complete the Lewis structure. (b) How many pi bonds…
A: Since you have posted a question with multiple sub-parts, we will solve the first three subparts for…
Q: n the Lewis model, the two bonds in a double bond look identical. However, valence bond theory shows…
A: In the Lewis model, the two bonds in a double bond look identical. However, valence bond theory…
Q: For the molecule benzene explain how to solve each of the following: a- draw the 6 molecular…
A: Benzene is an aromatic compound, with 12 sigma electrons (6 sigma bonds) & 6 pi electrons( 3 pi…
Q: angles a and b in the actual molecule of which this is a Lewis structure? H N C= N-H Note for…
A: Hybridization Bond angle sp 180° sp2 120° sp3 109°
Q: Explain the main postulate of the VSEPR model. List the five basegeometries (along with bond angles)…
A: Given: Explain the main postulate of the VSEPR model. List the five base geometries (along with bond…
Q: Get to know the general structure of an energy level diagram by labeling the follovwing. The…
A: Given energy-level diagram:
Q: One of the first drugs to be approved for use in treatment of acccquired immune deficiency syndrome…
A: Hii there, As there are multiple sub parts posted. we are answering first-three sub parts . If you…
Q: Please help answer this
A: The electrons used in the bond formations are the bonding electrons. The electrons that are not part…
Q: Indicate what kind of bonding orbital is formed when the following atomic or hybridized atomic…
A:
Q: C. What orbitals are overlapping to form the indicated bonds in each molecule. (Hint: Recall our…
A: Given structures are :
Q: What are the angles a and b in the actual molecule of which this is a Lewis structure? :0: Note for…
A: The given structure is lewis structure of formeldehyde. Looking at the lewis structure of…
Q: а. Lewis structure b. Drawing of shape (label angles) Name of shape С. d. Polar or nonpolar?, e.…
A: It is a primary amine and all atoms have SP3 hybridization so geometry is tetrahedral
Q: Do all of the following for each molecule or ion. a. Draw the Lewis Dot structure that minimizes…
A: Note: Since you have posted a question with multiple subparts, we will solve the first three…
Q: molecules that have been detected in interstellar space are HNCO What is the geometry at N in HNCO?…
A: Valence electrons on: H: 1 N: 5 C: 4 O: 6
Q: Which atomic orbitals overlap to form the C-Co (sigma) bonding molecular orbital indicated b the…
A: We have to tell which atomic orbitals overlap to form the C-C sigma bonding molecular orbital…
Q: сс с C-C=C C -C C-C= C a. Complete the formula, number the carbon atoms and name the molecule b.…
A:
Q: Use the References to access important values if needed for this question. Sigma Bonding A o bond…
A:
Q: 1. For the following molecules: 1) draw a Lewis Dot structure, including contributing resonance…
A: For HOCl, H - Atomic number = 1, Valency = 1 O - Atomic number = 8, Valency = 2 Cl- Atomic number =…
Q: 1. a) Draw the dominant Lewis structure for the allene molecule (1,2-propyl diene, CH2CCH2 ) and…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: 3. Consider the following molecule: B- -S-EN H Answer these questions about the molecule. If the…
A:
Q: Give the number and type of hybrid orbital that forms wheneach set of atomic orbitals mixes:(a) one…
A: The mixing of two atomic orbitals having the same energy level such that the orbital formed is a…
Q: 1. For the following molecules: 1) draw a Lewis Dot structure, including contributing resonance…
A: Valence Shell Electron Pair Repulsion (VSEPR) theory is used to predict the shape and molecular…
Q: which ones are non polar molecules? -Ncl3 -SO3 -PCL5
A: Which one of the following molecule is non polar ? : NCl3 , SO3 & PCl5
Q: Electron Groups (EGs) shape 2 Total smallest 90° 120° 180° number angle of bonded atoms shape (B.P)…
A: Hybridization is a hypothetical concept which involves mixing of orbitals to form new orbitals…
Q: For the following molecules: 1) draw a Lewis Dot structure, including contributing resonance…
A:
Q: Table of Molecules Moleculai # Electron # Bonding Electron Molecular P or NP Hybrid on Central Atom…
A: Given: Table. To find: complete the table Solution: Hybridization of the central metal atom and…
Q: 2) Determine the name, chemical formula; molar mass, and VSEPR molecular geometry of the compound…
A: The compound represented in the diagram has central atom with black color and two surrounding atoms…
Q: each molecule explain the error and suggest a correction. Make sure to include th drawing of the…
A: Given : We have to write hybridization for the given molecule.
Q: which one of the figures below has every sp3 hybridized atom circled?
A: Hybridization is based on the concept of mixing or atomic orbital to form new hybrid orbital and…
Q: Atomic Orbital #1 0.07 0.06 0.05 0.04 0.03 0.02 0.01 10 20 30 50 r (ao) Atomic Orbital #2 012 0.1…
A: The three-dimensional orientations of two orbitals are given and the probability distribution of…
Q: H C=C 0-C C-C H3C-o H ннон нннн H. —С-N-с-С-с-с-с—с-с-с-сНЗ H нннн H CH3 сapsaicin エー
A:
Q: I Bonding A T bond arises from "sideways" overlap of two parallel p orbitals. The electron density…
A: Carbon has 2 electrons in its P orbital in its ground state. The Carbon in C2H2 forms 1 sigma bond…
Q: i) AsF3, (ii) BrF3, (iii) ClO3-, (iv) BrO2- Answer the following questions for the molecules given…
A: Please find the attached images for the answer In this question I am giving the answer only for…
Q: In your Lewis structure for above, label all the bonds as sigma or pi. a) how many sigma and pi…
A:
Q: a. Determine the best Lewis structure representation for S2N2. Include the formal charges on each…
A: Since there are multiple sub parts in this question and it is not mentioned that which one has to be…
Q: CH3+ and CH3− are two highly reactive carbon species. a. What is the predicted hybridization and…
A: a) Since the CH3+ has a +ve charge hence it means the centre atom C has lost 1 valence electron and…
Q: Report all your results by using significant figures and use dimensional analysis. i) ASF3, (ii)…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Draw the dot structure for: a) ClO3-1 b) ICl2-1 c) What is the shape of ClO3-1? d) What is the shape of ICl2-1? e) What is the hybridization (sp, sp2, sp3, etc) about each central atom in parts a and b above?Consider the Lewis structure shown below. (a) Does the Lewis structure depict a neutral molecule or anion? If it is an ion, what is the charge on the ion? (b) What hybridizationis exhibited by each of the carbon atoms? (c) Arethere multiple equivalent resonance structures for the species?(d) How many electrons are in the π system of the species?Use sketches of orbitals to show how VB theory explains the bonding in the Cl2 molecule. Illustrate with appropriate orbital diagrams as well. Use these to determine the number of orbitals that overlap in the Cl2 molecule. How many orbitals overlap in Cl2? Choose answer that aplies. a. Bonding will be similar to the bonding in F2, because fluorine and chlorine are in the same group of the periodic table. b. One 3p orbital of each chlorine atom will participate in the bonding in the Cl2 molecule. c. One 2p orbital of each chlorine atom will participate in the bonding in the Cl2 molecule. d. Two sp7 molecular orbitals will participate in the bonding in Cl2 molecule. d. Bonding will be similar to the bonding in F2, because fluorine and chlorine are in the same period of the periodic table.
- One of the first drugs to be approved for use in treatment of acquired immune deficiency syndrome (AIDS) was azidothymidine (AZT). Complete the Lewis structure for AZT. a. How many carbon atoms are sp3 hybridized? b. How many carbon atoms are sp2 hybridized? c. Which atom is sp hybridized? d. How many σ bonds are in the molecule? e. How many π bonds are in the molecule? f. Wnat isthe N9N9N bond angle inthe azide (-N3) group? g. What is the H-Q-C bond angle in the side group attached to the five membered ring? h. What is the hybridization of the oxygen atom in the -CH2OH group?CH3+ and CH3− are two highly reactive carbon species. a. What is the predicted hybridization and geometry around each carbon atom? b.Two electrostatic potential plots are drawn for these species. Which ion corresponds to which diagram and why?Please fill in the blank ! In part B, the difference is that the molecules have more than one atom with two or more attached neighbors. As molecules become larger and more complex, VSEPR theory does not attempt to create names for the overall geometries that result. Instead, we continue to consider the geometry about one central atom at a time. By giving the arrangement about each such atom in the larger structure, we can generate enough information to develop an overall picture. Thus, in your report for part B, you should determine which atoms have more than one bound neighbor, then repeat the procedure for part A. When deciding whether the molecules in part B are polar or nonpolar, focus on the molecule as a whole, not the geometry about a single atom. If the molecule is polar, draw a dipole arrow on your sketch indicating polarity.
- In the hydrocarbon: (a) What is the hybridization at each carbon atom in themolecule? (b) How many σ bonds are there in the molecule?(c) How many π bonds? (d) Identify all the 120° bond anglesin the molecule.1, How many valence electrons does the molecule SO3 have? a, 24 b, 16 c, 18 d, 12 What's the electron geometry of SO3?The structure of the visual pigment retinal is shown in (1). Label each atom with its state of hybridization and specify the composition of each of the different type of bond.
- One of the first drugs to be approved for use in treatment of acccquired immune deficiency syndrome (AIDS) was azidothymidine (AZT). Complete the Lewis structure for AZT a. How many carbon atoms are sp3 hybridised? b. How many carbon atoms are sp2 hybridised? c. Which atom is sp hybridised? d. How many σ bonds are in the molecule? e. How many π bonds are in the molecule? f. What is the N9N9N bond angle in the azide (--N3) group? g. What is the H--Q--C bond angle in the side group attached to the five-membered ring? h. What is the hybridization of the oxygen atom in the --CH2OH group?The lactic acid molecule, CH3CH1OH2COOH, gives sourmilk its unpleasant, sour taste. (a) Draw the Lewis structurefor the molecule, assuming that carbon always forms fourbonds in its stable compounds. (b) How many p and howmany s bonds are in the molecule? (c) Which CO bond isshortest in the molecule? (d) What is the hybridization ofatomic orbitals around the carbon atom associated withthat short bond? (e) What are the approximate bond anglesaround each carbon atom in the molecule?Construct an approximate molecular orbital energy diagram for a hypothetical planar form of NH3. From a consideration of the atomic energy levels, place the N and H3 orbitals on either side of a molecular orbital energy-level diagram. Then use your judgement about the effect of bonding and anti bonding interactions and energies of the parent orbitals to construct the molecular orbital energy levels in the centre of your diagram and draw lines indicating the contributions of the atomic orbitals to each molecular orbital. Ionization energies are I(H1s) = 13.6 eV, I(N2s) = 26.0 eV, and I(N2p)=13.4 eV.

