10.124 Chlorine dioxide gas (CIO2) is used as a commercial bleach- ing agent. It bleaches materials by oxidizing them. In the course of these reactions, the CIO2 is itself reduced. (a) What is the Lewis structure for CLO2? (b) Why do you think that CIO2 is reduced so readily? (c) When a CIO2 molecule gains an electron, the chlorite ion, ClO2, forms. Draw the Lewis structure for CLO2. (d) Predict the O-Cl-Obond angle in the CIO2 ion. (e) One method of preparing ClO2 is by the reaction of chlorine and sodium chlorite 2 CIO2(8) + 2NaCl(s) Cl2(g) + 2 NaCIO2 (s) -
10.124 Chlorine dioxide gas (CIO2) is used as a commercial bleach- ing agent. It bleaches materials by oxidizing them. In the course of these reactions, the CIO2 is itself reduced. (a) What is the Lewis structure for CLO2? (b) Why do you think that CIO2 is reduced so readily? (c) When a CIO2 molecule gains an electron, the chlorite ion, ClO2, forms. Draw the Lewis structure for CLO2. (d) Predict the O-Cl-Obond angle in the CIO2 ion. (e) One method of preparing ClO2 is by the reaction of chlorine and sodium chlorite 2 CIO2(8) + 2NaCl(s) Cl2(g) + 2 NaCIO2 (s) -
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 86QRT
Related questions
Question
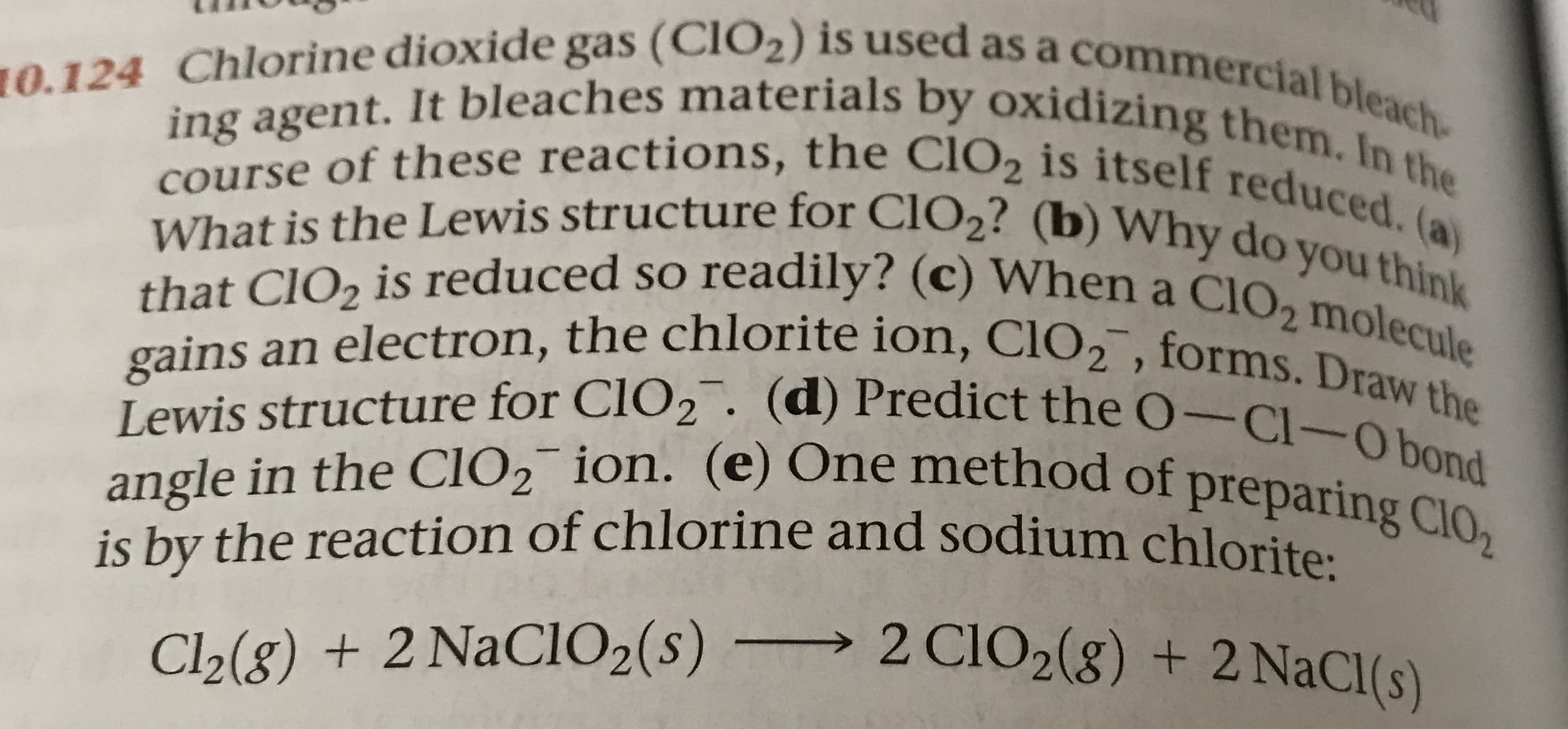
Transcribed Image Text:10.124 Chlorine dioxide gas (CIO2) is used as a commercial bleach-
ing agent. It bleaches materials by oxidizing them. In the
course of these reactions, the CIO2 is itself reduced. (a)
What is the Lewis structure for CLO2? (b) Why do you think
that CIO2 is reduced so readily? (c) When a CIO2 molecule
gains an electron, the chlorite ion, ClO2, forms. Draw the
Lewis structure for CLO2. (d) Predict the O-Cl-Obond
angle in the CIO2 ion. (e) One method of preparing ClO2
is by the reaction of chlorine and sodium chlorite
2 CIO2(8) + 2NaCl(s)
Cl2(g) + 2 NaCIO2 (s) -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
