100 10 0.1 Binding Energy (MJ/mol) The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom? Relative Number of Electrons
100 10 0.1 Binding Energy (MJ/mol) The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom? Relative Number of Electrons
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 96E: In the ground state of element 115, Uup, a. how many electrons have n = 5 as one of their quantum...
Related questions
Question
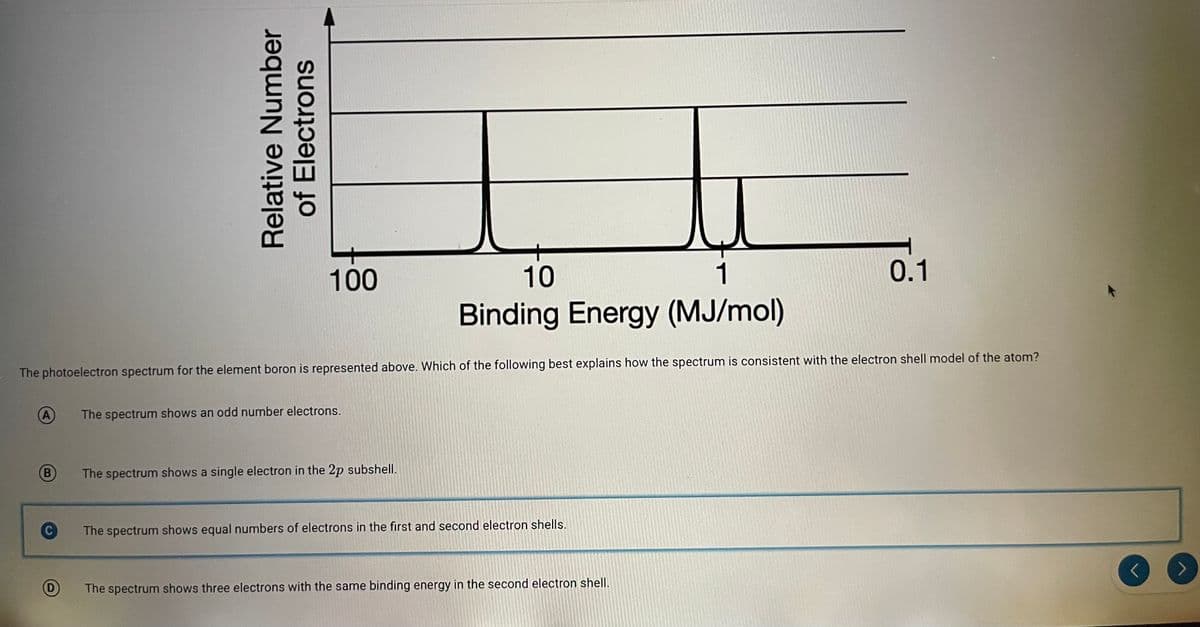
Transcribed Image Text:100
10
1
0.1
Binding Energy (MJ/mol)
The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom?
A
The spectrum shows an odd number electrons.
B
The spectrum shows a single electron in the 2p subshell.
C
The spectrum shows equal numbers of electrons in the first and second electron shells.
D)
The spectrum shows three electrons with the same binding energy in the second electron shell.
Relative Number
of Electrons
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning