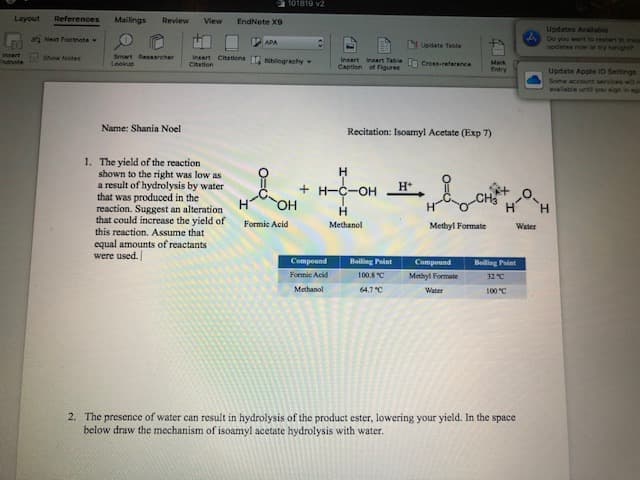
101819 v2 Layout References Mailings Review View EndNete X9 Updates Available Do you want to restart to ira uodates now or try tonic ah Next Footnote APA Update Tabla Insart ndnote Smart Resaarcher Leokup Citations blegrachy Show Notes insert Citation insart Tabie tn Cress-reerence Intert Mark Caption of Flgures Entry Update Apple ID Settings Some account services witno available untl you sign in gm Name: Shania Noel Recitation: Isoamyl Acetate (Exp 7) 1. The yield of the reaction shown to the right was low as a result of hydrolysis by water that was produced in the reaction. Suggest an alteration that could increase the yield of this reaction. Assume that equal amounts of reactants Н н +H-C-OH ОН н н Н н Formic Acid Methanol Methyl Formate Water were used. Compound Boiling Point Compound Beling Point Foemic Acid 100.8C Methyl Formate 32 "C Methanol 64.7C Water 100"C 2. The presence of water can result in hydrolysis of the product ester, lowering your yield. In the space below draw the mechanism of isoamyl acetate hydrolysis with water
101819 v2 Layout References Mailings Review View EndNete X9 Updates Available Do you want to restart to ira uodates now or try tonic ah Next Footnote APA Update Tabla Insart ndnote Smart Resaarcher Leokup Citations blegrachy Show Notes insert Citation insart Tabie tn Cress-reerence Intert Mark Caption of Flgures Entry Update Apple ID Settings Some account services witno available untl you sign in gm Name: Shania Noel Recitation: Isoamyl Acetate (Exp 7) 1. The yield of the reaction shown to the right was low as a result of hydrolysis by water that was produced in the reaction. Suggest an alteration that could increase the yield of this reaction. Assume that equal amounts of reactants Н н +H-C-OH ОН н н Н н Formic Acid Methanol Methyl Formate Water were used. Compound Boiling Point Compound Beling Point Foemic Acid 100.8C Methyl Formate 32 "C Methanol 64.7C Water 100"C 2. The presence of water can result in hydrolysis of the product ester, lowering your yield. In the space below draw the mechanism of isoamyl acetate hydrolysis with water
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.82PAE: Use the web to research the elastic modulus and yield strength of carbon fiber composites. How do...
Related questions
Question
Transcribed Image Text:101819 v2
Layout
References
Mailings
Review
View
EndNete X9
Updates Available
Do you want to restart to ira
uodates now or try tonic
ah Next Footnote
APA
Update Tabla
Insart
ndnote
Smart Resaarcher
Leokup
Citations blegrachy
Show Notes
insert
Citation
insart Tabie tn Cress-reerence
Intert
Mark
Caption of Flgures
Entry
Update Apple ID Settings
Some account services witno
available untl you sign in gm
Name: Shania Noel
Recitation: Isoamyl Acetate (Exp 7)
1. The yield of the reaction
shown to the right was low as
a result of hydrolysis by water
that was produced in the
reaction. Suggest an alteration
that could increase the yield of
this reaction. Assume that
equal amounts of reactants
Н
н
+H-C-OH
ОН
н
н
Н
н
Formic Acid
Methanol
Methyl Formate
Water
were used.
Compound
Boiling Point
Compound
Beling Point
Foemic Acid
100.8C
Methyl Formate
32 "C
Methanol
64.7C
Water
100"C
2. The presence of water can result in hydrolysis of the product ester, lowering your yield. In the space
below draw the mechanism of isoamyl acetate hydrolysis with water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning