10-27. A 10.231-g sample of window cleaner containing ammonia was diluted with 39.466 g of water. Then 4.373 g of solution were titrated with 14.22 mL of 0.106 3 M HCl to reach a bromocresol green end point. (a) What fraction of the 10.231-g sample of window cleaner is contained in the 4.373 g that were analyzed? (b) How many grams of NH3 (FM 17.031) were in the 4.373-g sample? (c) Find the weight percent of NH3 in the cleaner.
10-27. A 10.231-g sample of window cleaner containing ammonia was diluted with 39.466 g of water. Then 4.373 g of solution were titrated with 14.22 mL of 0.106 3 M HCl to reach a bromocresol green end point. (a) What fraction of the 10.231-g sample of window cleaner is contained in the 4.373 g that were analyzed? (b) How many grams of NH3 (FM 17.031) were in the 4.373-g sample? (c) Find the weight percent of NH3 in the cleaner.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.20QAP
Related questions
Question
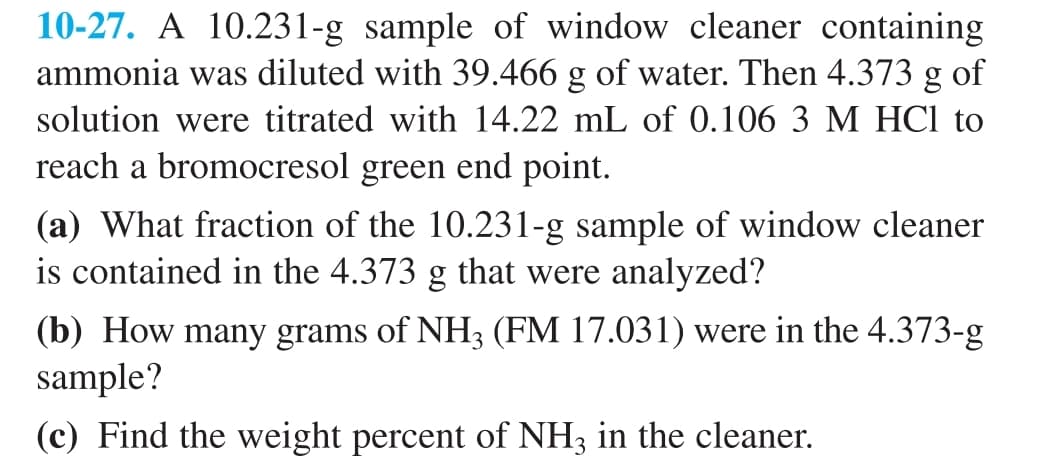
Transcribed Image Text:10-27. A 10.231-g sample of window cleaner containing
ammonia was diluted with 39.466 g of water. Then 4.373 g of
solution were titrated with 14.22 mL of 0.106 3 M HCl to
reach a bromocresol green end point.
(a) What fraction of the 10.231-g sample of window cleaner
is contained in the 4.373 g that were analyzed?
(b) How many grams of NH3 (FM 17.031) were in the 4.373-g
sample?
(c) Find the weight percent of NH3 in the cleaner.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you



