11 of 1 Review I Constants Periodic T Butane, C4H10, reacts with oxygen, O2, to form water, H2O, and carbon dioxide, CO2, as shown in the following chemical equation: CUipouu s. 2. Convert from moles of compound X to moles of compound Y using the coefficients in the balanced chemical equation. 3. Convert from moles of compound Y to grams of compound Y using the molar mass of compound Y. 2C4H10(g)1302(g)10H2O(g) +8CO2 (g) The coefficients in this equation represent mole ratios. Notice that the coefficient for water (10) is five times that of butane (2). Thus, the number of moles of water produced is five times the number of moles Part B of butane that react. Calculate the mass of water produced when 6.96 g of butane reacts with excess oxygen. Also, notice that the coefficient for butane (2) is one- fourth the coefficient of carbon dioxide (8). Thus, the Express your answer to three significant figures and include the appropriate units. number of moles of butane that react is one-fourth View Available Hint(s) the number of moles of carbon dioxide that you produce. But be careful! If you are given the mass of a compound, you must first convert to moles before applying these ratios. HA ? Units Value Submit Part C P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy PolicyI Permissions | Contact Us F12 FII F10 F9 F8
11 of 1 Review I Constants Periodic T Butane, C4H10, reacts with oxygen, O2, to form water, H2O, and carbon dioxide, CO2, as shown in the following chemical equation: CUipouu s. 2. Convert from moles of compound X to moles of compound Y using the coefficients in the balanced chemical equation. 3. Convert from moles of compound Y to grams of compound Y using the molar mass of compound Y. 2C4H10(g)1302(g)10H2O(g) +8CO2 (g) The coefficients in this equation represent mole ratios. Notice that the coefficient for water (10) is five times that of butane (2). Thus, the number of moles of water produced is five times the number of moles Part B of butane that react. Calculate the mass of water produced when 6.96 g of butane reacts with excess oxygen. Also, notice that the coefficient for butane (2) is one- fourth the coefficient of carbon dioxide (8). Thus, the Express your answer to three significant figures and include the appropriate units. number of moles of butane that react is one-fourth View Available Hint(s) the number of moles of carbon dioxide that you produce. But be careful! If you are given the mass of a compound, you must first convert to moles before applying these ratios. HA ? Units Value Submit Part C P Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy PolicyI Permissions | Contact Us F12 FII F10 F9 F8
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 44AP: A possible practical way to eliminate oxides of nitrogen(such as NO2 ) from automobile exhaust gases...
Related questions
Question
100%
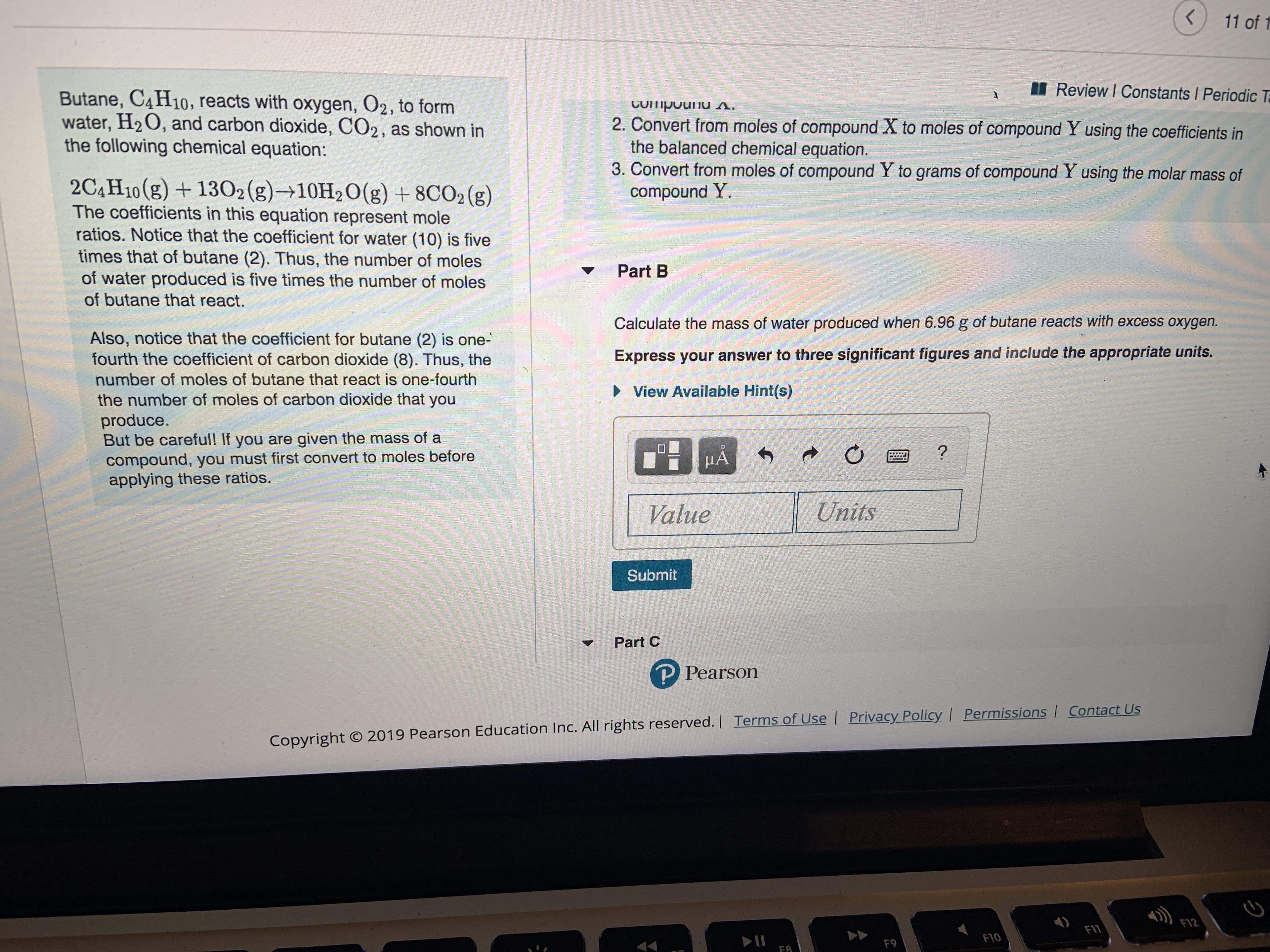
Transcribed Image Text:11 of 1
Review I Constants Periodic T
Butane, C4H10, reacts with oxygen, O2, to form
water, H2O, and carbon dioxide, CO2, as shown in
the following chemical equation:
CUipouu s.
2. Convert from moles of compound X to moles of compound Y using the coefficients in
the balanced chemical equation.
3. Convert from moles of compound Y to grams of compound Y using the molar mass of
compound Y.
2C4H10(g)1302(g)10H2O(g) +8CO2 (g)
The coefficients in this equation represent mole
ratios. Notice that the coefficient for water (10) is five
times that of butane (2). Thus, the number of moles
of water produced is five times the number of moles
Part B
of butane that react.
Calculate the mass of water produced when 6.96 g of butane reacts with excess oxygen.
Also, notice that the coefficient for butane (2) is one-
fourth the coefficient of carbon dioxide (8). Thus, the
Express your answer to three significant figures and include the appropriate units.
number of moles of butane that react is one-fourth
View Available Hint(s)
the number of moles of carbon dioxide that you
produce.
But be careful! If you are given the mass of a
compound, you must first convert to moles before
applying these ratios.
HA
?
Units
Value
Submit
Part C
P Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy PolicyI Permissions | Contact Us
F12
FII
F10
F9
F8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div