11.38 (a) Would you expect the viscosity of n-pentane, CH3CH2CH2CH2CH3, to be larger or smaller than the vis- cosity of n-hexane, CH3CH2CH2CH2CH2CH3? (b) Would you expect the viscosity of neopentane, (CH3)4C, to be smaller or larger than the viscosity of n-pentane? (See Fig- ure 11.6 to see the shapes of these molecules.) Linear molecule larger Surface area enhances intermolecular contact and increases dispersion force. n-Pentane (CsH12) 309.4 K bp Spherical molecule-smaller surface area diminishes intermolecular contact and decreases dispersion force. Neopentane (C5H12) bp = 282.7 K Figure 11.6 Molecular shape affects intermolecular attraction. Molecules of n-pentane make more contact with each other than do neopentane molecules. Thus, n-pentane has stronger intermolecular attractive forces and therefore a higher boiling point.
11.38 (a) Would you expect the viscosity of n-pentane, CH3CH2CH2CH2CH3, to be larger or smaller than the vis- cosity of n-hexane, CH3CH2CH2CH2CH2CH3? (b) Would you expect the viscosity of neopentane, (CH3)4C, to be smaller or larger than the viscosity of n-pentane? (See Fig- ure 11.6 to see the shapes of these molecules.) Linear molecule larger Surface area enhances intermolecular contact and increases dispersion force. n-Pentane (CsH12) 309.4 K bp Spherical molecule-smaller surface area diminishes intermolecular contact and decreases dispersion force. Neopentane (C5H12) bp = 282.7 K Figure 11.6 Molecular shape affects intermolecular attraction. Molecules of n-pentane make more contact with each other than do neopentane molecules. Thus, n-pentane has stronger intermolecular attractive forces and therefore a higher boiling point.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 45SCQ: Liquid ethylene glycol, HOCH2CH2OH, is one of the main ingredients in commercial antifreeze. Would...
Related questions
Question
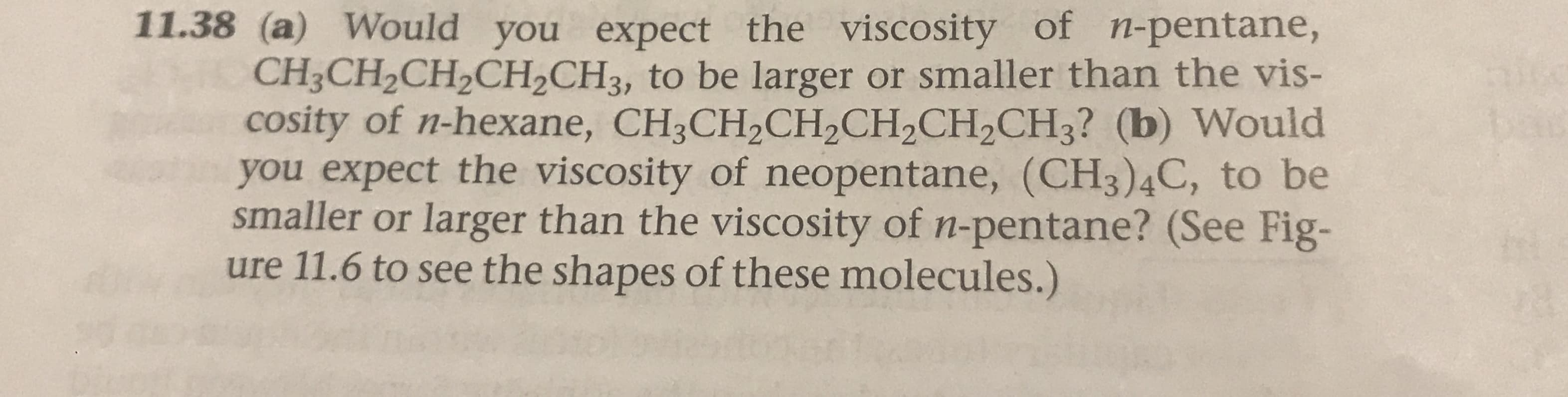
Transcribed Image Text:11.38 (a) Would you expect the viscosity of n-pentane,
CH3CH2CH2CH2CH3, to be larger or smaller than the vis-
cosity of n-hexane, CH3CH2CH2CH2CH2CH3? (b) Would
you expect the viscosity of neopentane, (CH3)4C, to be
smaller or larger than the viscosity of n-pentane? (See Fig-
ure 11.6 to see the shapes of these molecules.)
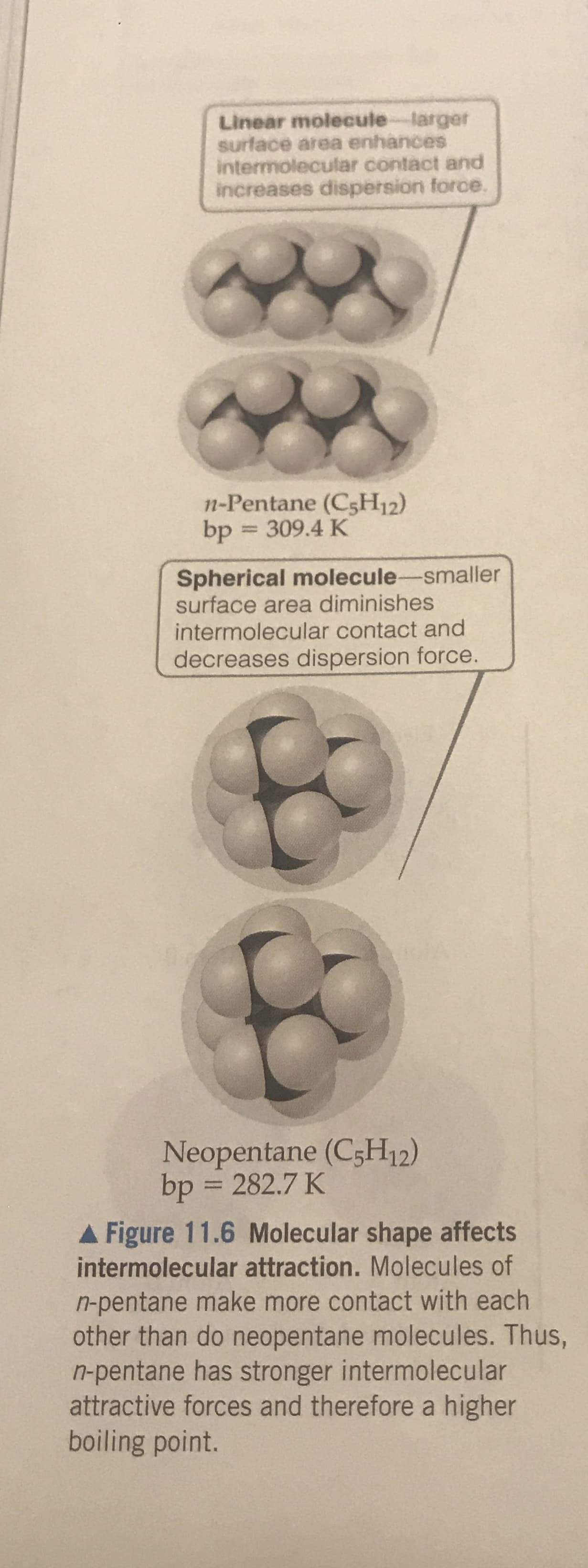
Transcribed Image Text:Linear molecule larger
Surface area enhances
intermolecular contact and
increases dispersion force.
n-Pentane (CsH12)
309.4 K
bp
Spherical molecule-smaller
surface area diminishes
intermolecular contact and
decreases dispersion force.
Neopentane (C5H12)
bp = 282.7 K
Figure 11.6 Molecular shape affects
intermolecular attraction. Molecules of
n-pentane make more contact with each
other than do neopentane molecules. Thus,
n-pentane has stronger intermolecular
attractive forces and therefore a higher
boiling point.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning