11.61 The phase diagram for neon is blupl Liquid Supercritical 103 Stio nobtoin3o 102 fluid bpszs stlq YO bo Snss Solid Critical point 10 ba tpojczpsti1 10 1T 1 Triple point 9 5dmg Gas 10 2 ei b 10 3 20 40 60 80 100 entille Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will solid neon sub- lime? (c) At room temperature (T = 25 °C) can neon be liq- uefied by compressing it? wo paug Pressure (atm)
11.61 The phase diagram for neon is blupl Liquid Supercritical 103 Stio nobtoin3o 102 fluid bpszs stlq YO bo Snss Solid Critical point 10 ba tpojczpsti1 10 1T 1 Triple point 9 5dmg Gas 10 2 ei b 10 3 20 40 60 80 100 entille Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will solid neon sub- lime? (c) At room temperature (T = 25 °C) can neon be liq- uefied by compressing it? wo paug Pressure (atm)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 43QRT
Related questions
Question
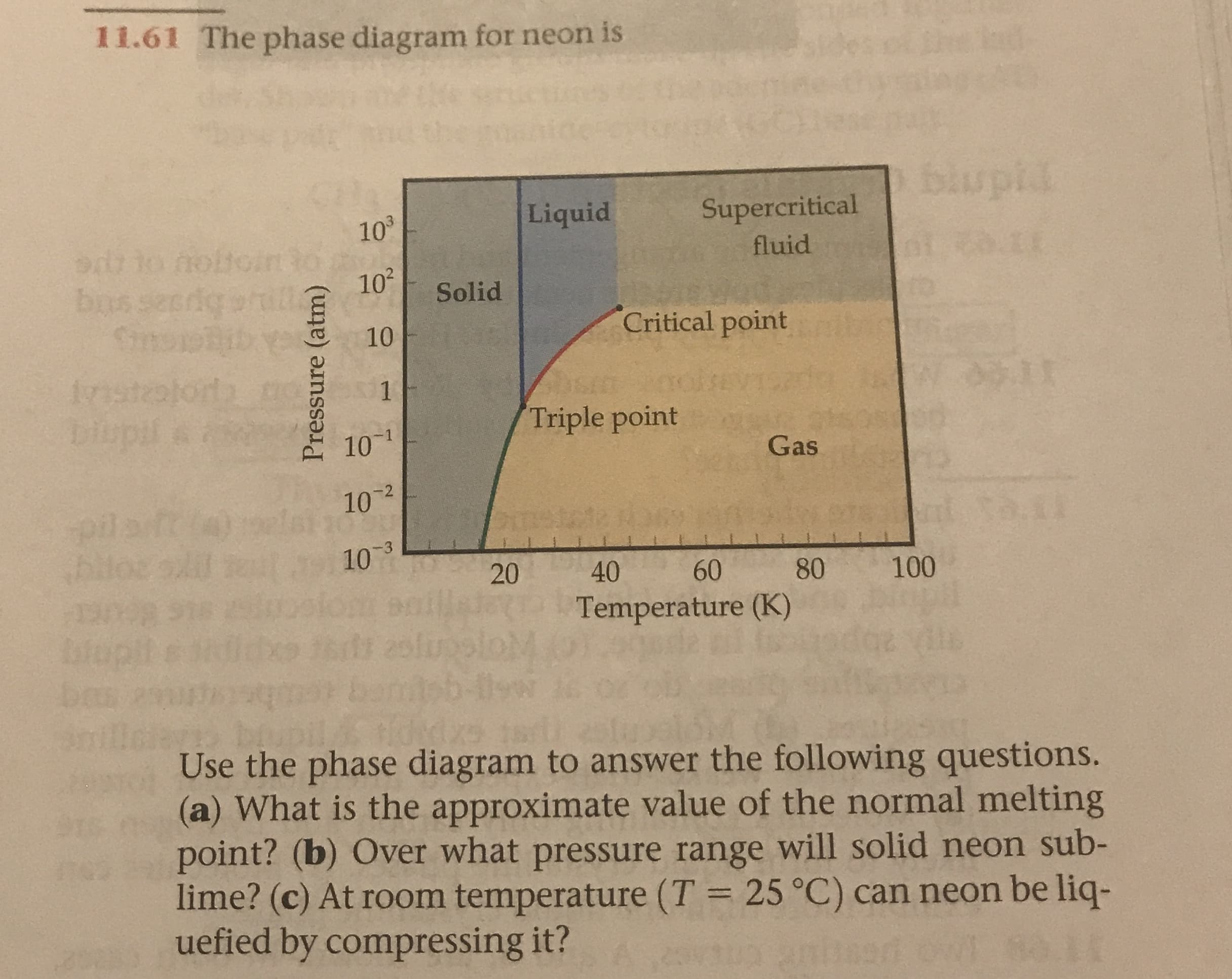
Transcribed Image Text:11.61 The phase diagram for neon is
blupl
Liquid
Supercritical
103
Stio nobtoin3o
102
fluid
bpszs stlq
YO
bo
Snss
Solid
Critical point
10
ba tpojczpsti1
10
1T
1
Triple point
9 5dmg
Gas
10 2
ei
b
10 3
20
40
60
80
100
entille
Temperature (K)
Use the phase diagram to answer the following questions.
(a) What is the approximate value of the normal melting
point? (b) Over what pressure range will solid neon sub-
lime? (c) At room temperature (T = 25 °C) can neon be liq-
uefied by compressing it?
wo paug
Pressure (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning