11.8 At three different temperatures, Ti, T, and T, the molecules in a liquid crystal align in these ways: 2.1 5EL6 Тз T2 T1 (a) At which temperature or temperatures is the substance in a liquid crystalline state? At those temperatures, which type of liquid crystalline phase is depicted? (b) Which is the highest of these three temperatures? [Section 11.7)
11.8 At three different temperatures, Ti, T, and T, the molecules in a liquid crystal align in these ways: 2.1 5EL6 Тз T2 T1 (a) At which temperature or temperatures is the substance in a liquid crystalline state? At those temperatures, which type of liquid crystalline phase is depicted? (b) Which is the highest of these three temperatures? [Section 11.7)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 8E: The types of intermolecular forces in a substance are identical whether it is a solid, a liquid, or...
Related questions
Question
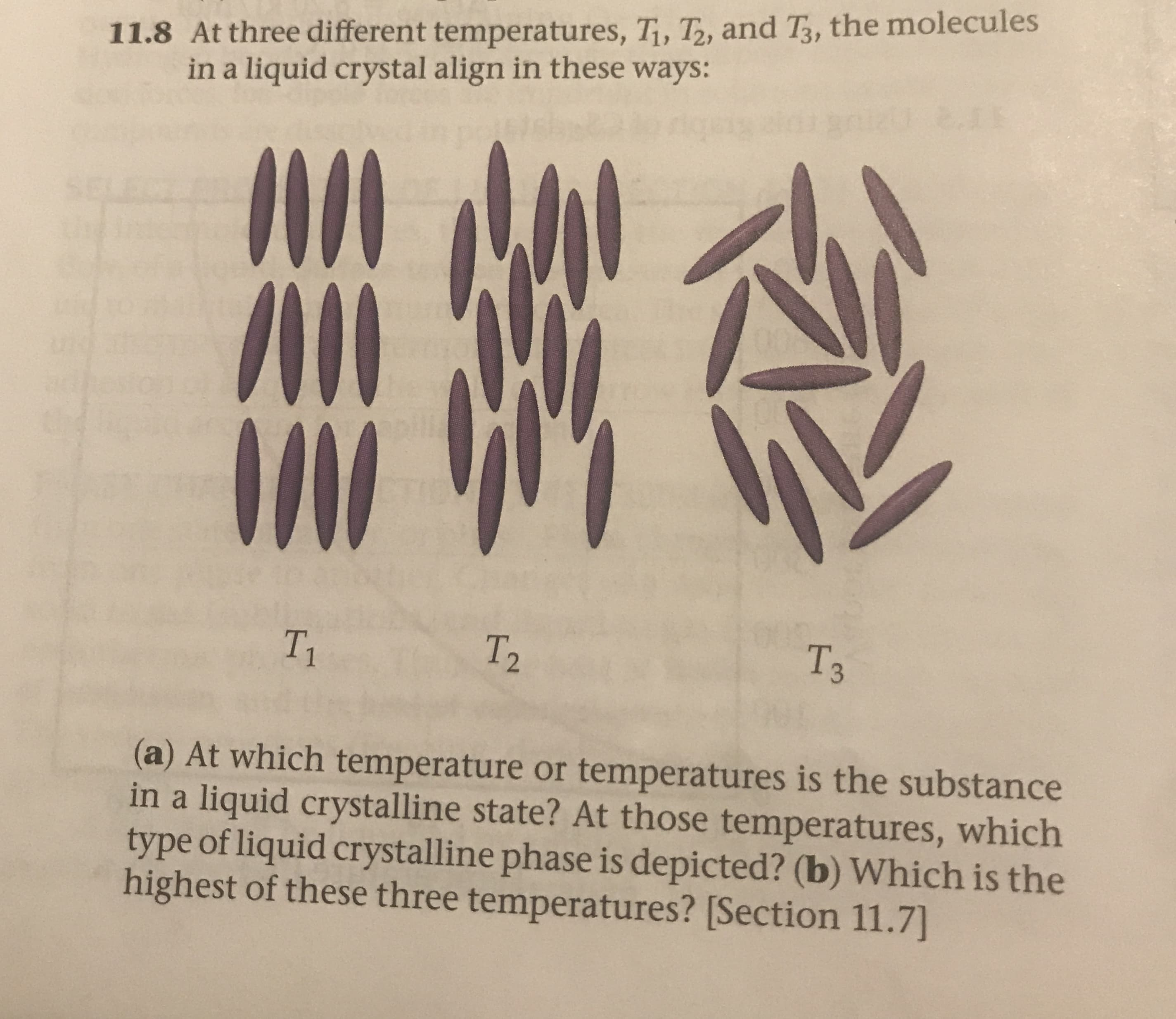
Transcribed Image Text:11.8 At three different temperatures, Ti, T, and T, the molecules
in a liquid crystal align in these ways:
2.1
5EL6
Тз
T2
T1
(a) At which temperature or temperatures is the substance
in a liquid crystalline state? At those temperatures, which
type of liquid crystalline phase is depicted? (b) Which is the
highest of these three temperatures? [Section 11.7)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning