115 110 105 100 1400 1300 C 1200 I100 1000 900 85 80 75 0 0.2 0.4 0.6 0.8 1.0 Molc fraction benzene P-1 atm (a) Tay diagram 0 0.2 0.4 0.6 0.8 1.0 Mole fraction benzenc T-100°C (b) Pxy diagram
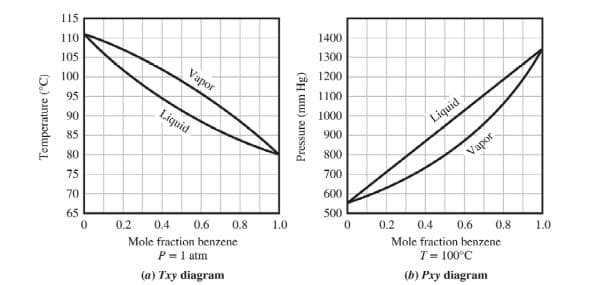
The T-x-y and P-x-y diagrams for the binary benzene-toluene system is shown below.
a. The bubble point temperature for a vapor-liquid equilibrium system of 80% benzene and 20% toluene is about 83°C. Using Antoine’s Equation and without using the diagrams, calculate the composition of the vapor that is generated at 83°C.
b. This time, using the diagrams provided, determine the answer for Part a.
c. A vapor at atmospheric pressure consisting of 80% benzene and 20% toluene has a dew point of 88°C. Using Antoine’s Equation and without using the diagrams, calculate the composition of the liquid that is in equilibrium with the vapor.
d. Using the diagrams provided, what is the composition of the liquid that is in equilibrium with a vapor that is 50% benzene and 50% toluene?
e. You have a gas mixture of 40% benzene, 10% toluene, and 50% helium. The gas mixture is compressed isothermally at 80°C until condensation occurs. The helium is insoluble in the condensate. Without using the diagrams, determine the pressure at which condensation begins and the composition of the initial condensate.
* Answer a, b, c please.
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images









