12. While each molecule below has more protons (H-atoms) than being shown, just focus on the protons identified. Compare the two protons identified. 1) Draw the two conjugate bases that could possibly result (althou gh one will be preferred over the other). The purpose here is to make you look at the conjugate base and the atom upon which the negative charge resides, then you should consider your SERIO factors. 2) Identify which proton is more acidic by circling the H, or H, on the original molecule. 3) Explain why by comparing the conjugate bases. Cirele the factor you considered when comparing the stabilities of the conjugate bases. Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization СвА св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization св, св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization O-He CBA св, Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization св, св, Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization он, Н,о свА св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization св, св,
12. While each molecule below has more protons (H-atoms) than being shown, just focus on the protons identified. Compare the two protons identified. 1) Draw the two conjugate bases that could possibly result (althou gh one will be preferred over the other). The purpose here is to make you look at the conjugate base and the atom upon which the negative charge resides, then you should consider your SERIO factors. 2) Identify which proton is more acidic by circling the H, or H, on the original molecule. 3) Explain why by comparing the conjugate bases. Cirele the factor you considered when comparing the stabilities of the conjugate bases. Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization СвА св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization св, св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization O-He CBA св, Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization св, св, Explanation Size Electronegativity Resonance Inductive effect Orbital hybridization он, Н,о свА св, Explanation: Size Electronegativity Resonance Inductive effect Orbital hybridization св, св,
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 17E
Related questions
Question
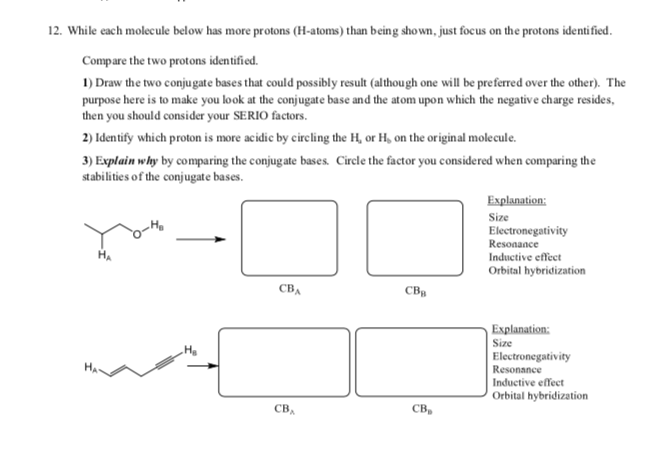
Transcribed Image Text:12. While each molecule below has more protons (H-atoms) than being shown, just focus on the protons identified.
Compare the two protons identified.
1) Draw the two conjugate bases that could possibly result (althou gh one will be preferred over the other). The
purpose here is to make you look at the conjugate base and the atom upon which the negative charge resides,
then you should consider your SERIO factors.
2) Identify which proton is more acidic by circling the H, or H, on the original molecule.
3) Explain why by comparing the conjugate bases. Cirele the factor you considered when comparing the
stabilities of the conjugate bases.
Explanation:
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
СвА
св,
Explanation:
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
св,
св,
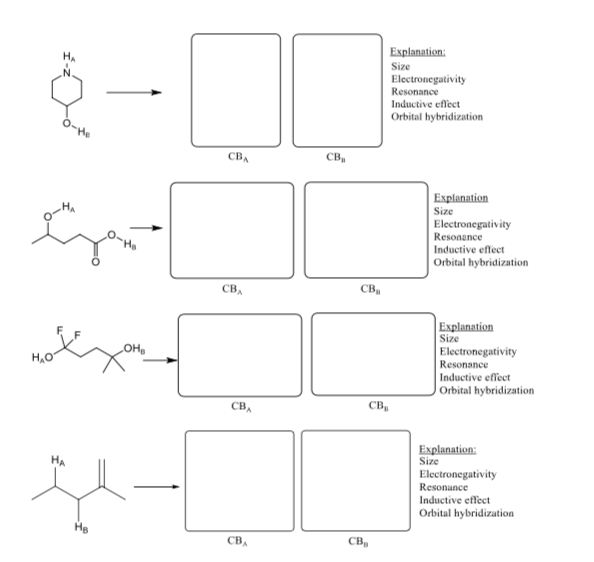
Transcribed Image Text:Explanation:
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
O-He
CBA
св,
Explanation
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
св,
св,
Explanation
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
он,
Н,о
свА
св,
Explanation:
Size
Electronegativity
Resonance
Inductive effect
Orbital hybridization
св,
св,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole