Chapter3: Mechanisms
Section: Chapter Questions
Problem 143EQ
Related questions
Question
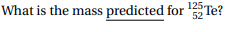
Transcribed Image Text:125Te?
Expert Solution
Step 1
The given isotopic notion refers to Tellurium- 125.
The atomic number = 52 and
The mass number = 125.
Step 2
The mass number refers to the number of protons and neutrons in the given atom. The mass of these protons and neutrons in an atom is considered as atomic mass.
So, find the number of protons and neutrons in the given atom:
The atomic number = number protons = 52
The number of neutrons = mass number – atomic number
= 125 – 52 = 73
Step by step
Solved in 3 steps

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning