13. Consider the following pictures of gases at the same temperature. O Ne and -Ar = Ar . 1V. V. V1 vii. Arrange each of these pictures in order from lowest to highest (note: in some cases the pictures may be equal in ranking) a. pressure b. average kinetic energy c. density d. root mean square speed Assume the large box is twice the volume of the small box. Approximate the mass of the argon and neon atoms to 2 significant figures.
13. Consider the following pictures of gases at the same temperature. O Ne and -Ar = Ar . 1V. V. V1 vii. Arrange each of these pictures in order from lowest to highest (note: in some cases the pictures may be equal in ranking) a. pressure b. average kinetic energy c. density d. root mean square speed Assume the large box is twice the volume of the small box. Approximate the mass of the argon and neon atoms to 2 significant figures.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 30Q
Related questions
Question
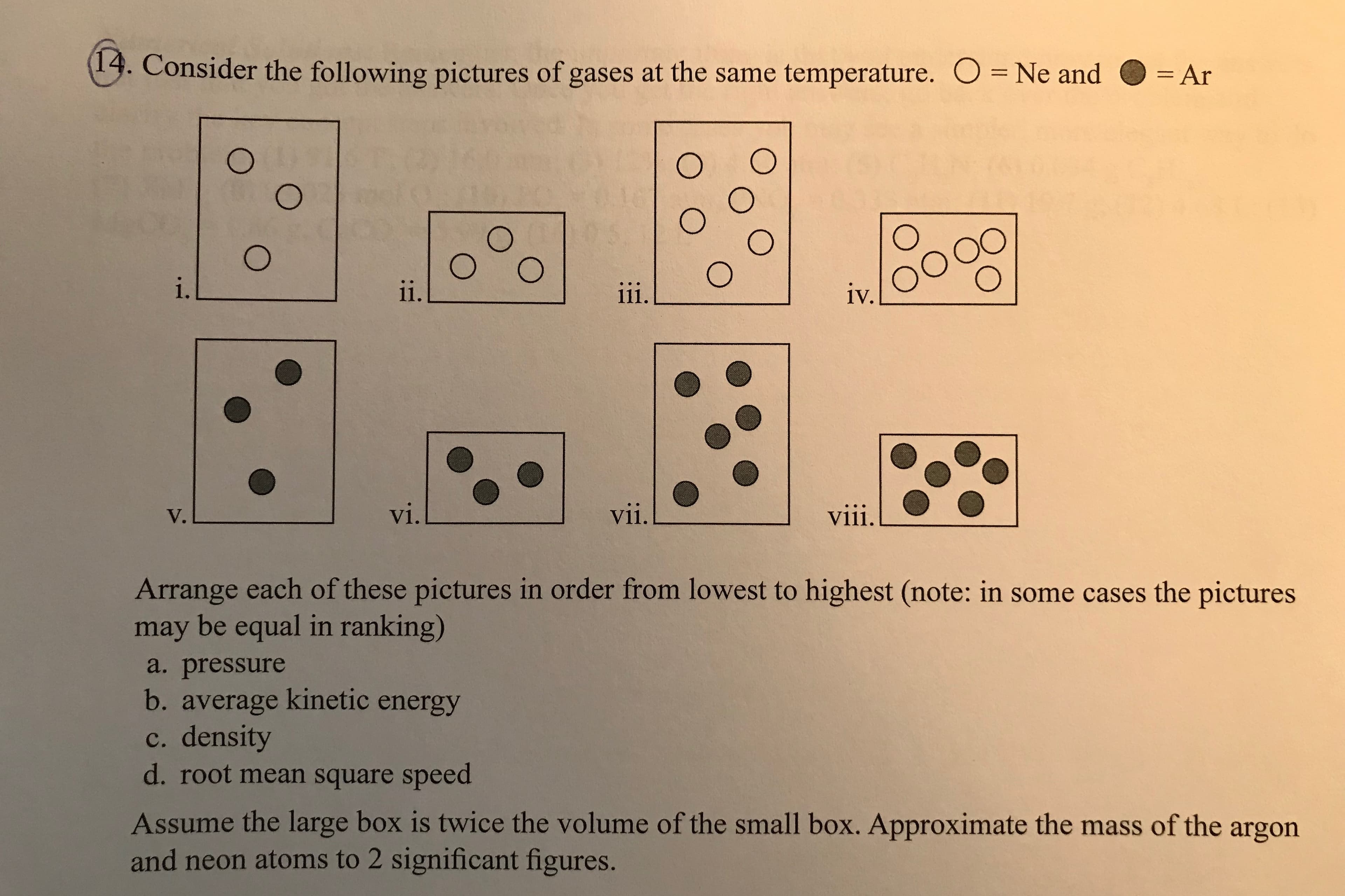
Transcribed Image Text:13. Consider the following pictures of gases at the same temperature.
O Ne and -Ar
= Ar
.
1V.
V.
V1
vii.
Arrange each of these pictures in order from lowest to highest (note: in some cases the pictures
may be equal in ranking)
a. pressure
b. average kinetic energy
c. density
d. root mean square speed
Assume the large box is twice the volume of the small box. Approximate the mass of the argon
and neon atoms to 2 significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax