14. A 10.0-g sample of water requires 418 J of energy to increase in temperature by 10°C, whereas a 10.0-g sample of copper requires 38.5 J of energy to increase in temperature by the same amount. Explain the concept that relates to this difference. What is it called? How does it relate?
14. A 10.0-g sample of water requires 418 J of energy to increase in temperature by 10°C, whereas a 10.0-g sample of copper requires 38.5 J of energy to increase in temperature by the same amount. Explain the concept that relates to this difference. What is it called? How does it relate?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 37QAP: The “Chemistry in Focus” segment Nature Has Hot Plants discusses thermogenic, or heat-producing,...
Related questions
Question
Please answer it. Thanks!
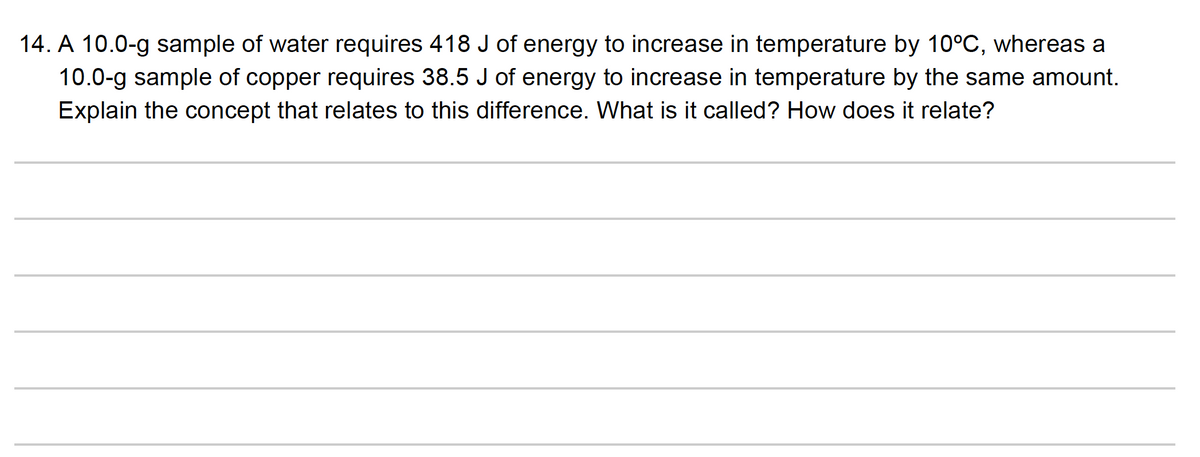
Transcribed Image Text:14. A 10.0-g sample of water requires 418 J of energy to increase in temperature by 10°C, whereas a
10.0-g sample of copper requires 38.5 J of energy to increase in temperature by the same amount.
Explain the concept that relates to this difference. What is it called? How does it relate?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning