14.1 Heat In Work, Energy, and Energy Resources, we defined work as force times distance and learned that work done on an object changes its kinetic energy. We also saw in Temperature, Kinetic Theory, and the Gas Laws that temperature is proportional to the (average) kinetic energy of atoms and molecules. We say that a thermal system has a certain internal energy: its internal energy is higher if the temperature is higher. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter to the colder object until equilibrium is reached and the bodies reach thermal equilibrium (i.e., they are at the same temperature). No work is done by either object, because no force acts through a distance. The transfer of energy is caused by the temperature difference, and ceases once the temperatures are equal. These observations lead to the following definition of heat Heat is the spontaneous transfer of energy due to a temperature difference. As noted in Temperature, Kinetic Theory, and the Gas Laws, heat is often confused with temperature. For example, we may say the heat was unbearable, when we actually mean that the temperature was high. Heat is a form of energy, whereas temperature is not. The misconception arises because we are sensitive to the flow of heat, rather than the temperature. Owing to the fact that heat is a form of energy, it has the Sl unit of joule (J). The calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00°C –specifically, between 14.5°C and 15.5C. since there is a slight temperature dependence. Perhaps the most common unit of heat is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by 1.00°C. Since mass is most often specified in kilograms. kilocalorie is commonly used. Food calories (given the notation Cal, and sometimes called "big calorie") are actually kilocalories ( 1 kilocalorie = 1000 calories ), a fact not easily determined from package labeling. (a) T' Figure 14.2 In figure (a) the soft drinik and the ice have different temperatures, 71 and T2. and are not in thermal equilibrium. In figure (b), when the soft drink and ice are allowed to interact, energy is transferred until they reach the same temperature /'.achieving equilibrium. Heat transfer occurs due to the difference in temperatures. In fact, since the soft drink and ice are both in contact with the surrounding air and bench, the equilibrium temperature will be the same for both.
14.1 Heat In Work, Energy, and Energy Resources, we defined work as force times distance and learned that work done on an object changes its kinetic energy. We also saw in Temperature, Kinetic Theory, and the Gas Laws that temperature is proportional to the (average) kinetic energy of atoms and molecules. We say that a thermal system has a certain internal energy: its internal energy is higher if the temperature is higher. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter to the colder object until equilibrium is reached and the bodies reach thermal equilibrium (i.e., they are at the same temperature). No work is done by either object, because no force acts through a distance. The transfer of energy is caused by the temperature difference, and ceases once the temperatures are equal. These observations lead to the following definition of heat Heat is the spontaneous transfer of energy due to a temperature difference. As noted in Temperature, Kinetic Theory, and the Gas Laws, heat is often confused with temperature. For example, we may say the heat was unbearable, when we actually mean that the temperature was high. Heat is a form of energy, whereas temperature is not. The misconception arises because we are sensitive to the flow of heat, rather than the temperature. Owing to the fact that heat is a form of energy, it has the Sl unit of joule (J). The calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by 1.00°C –specifically, between 14.5°C and 15.5C. since there is a slight temperature dependence. Perhaps the most common unit of heat is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by 1.00°C. Since mass is most often specified in kilograms. kilocalorie is commonly used. Food calories (given the notation Cal, and sometimes called "big calorie") are actually kilocalories ( 1 kilocalorie = 1000 calories ), a fact not easily determined from package labeling. (a) T' Figure 14.2 In figure (a) the soft drinik and the ice have different temperatures, 71 and T2. and are not in thermal equilibrium. In figure (b), when the soft drink and ice are allowed to interact, energy is transferred until they reach the same temperature /'.achieving equilibrium. Heat transfer occurs due to the difference in temperatures. In fact, since the soft drink and ice are both in contact with the surrounding air and bench, the equilibrium temperature will be the same for both.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter15: Thermodynamics
Section: Chapter Questions
Problem 45PE: A 4ton air conditioner removes 5.60107J (48,000 British thermal units) from a cold environment in...
Related questions
Question
Heat
• Define
Transcribed Image Text:14.1 Heat
In Work, Energy, and Energy Resources, we defined work as force times distance and learned that work done on an object
changes its kinetic energy. We also saw in Temperature, Kinetic Theory, and the Gas Laws that temperature is proportional to
the (average) kinetic energy of atoms and molecules. We say that a thermal system has a certain internal energy: its internal
energy is higher if the temperature is higher. If two objects at different temperatures are brought in contact with each other,
energy is transferred from the hotter to the colder object until equilibrium is reached and the bodies reach thermal equilibrium
(i.e., they are at the same temperature). No work is done by either object, because no force acts through a distance. The transfer
of energy is caused by the temperature difference, and ceases once the temperatures are equal. These observations lead to the
following definition of heat Heat is the spontaneous transfer of energy due to a temperature difference.
As noted in Temperature, Kinetic Theory, and the Gas Laws, heat is often confused with temperature. For example, we may
say the heat was unbearable, when we actually mean that the temperature was high. Heat is a form of energy, whereas
temperature is not. The misconception arises because we are sensitive to the flow of heat, rather than the temperature.
Owing to the fact that heat is a form of energy, it has the Sl unit of joule (J). The calorie (cal) is a common unit of energy, defined
as the energy needed to change the temperature of 1.00 g of water by 1.00°C –specifically, between 14.5°C and 15.5C.
since there is a slight temperature dependence. Perhaps the most common unit of heat is the kilocalorie (kcal), which is the
energy needed to change the temperature of 1.00 kg of water by 1.00°C. Since mass is most often specified in kilograms.
kilocalorie is commonly used. Food calories (given the notation Cal, and sometimes called "big calorie") are actually kilocalories (
1 kilocalorie = 1000 calories ), a fact not easily determined from package labeling.
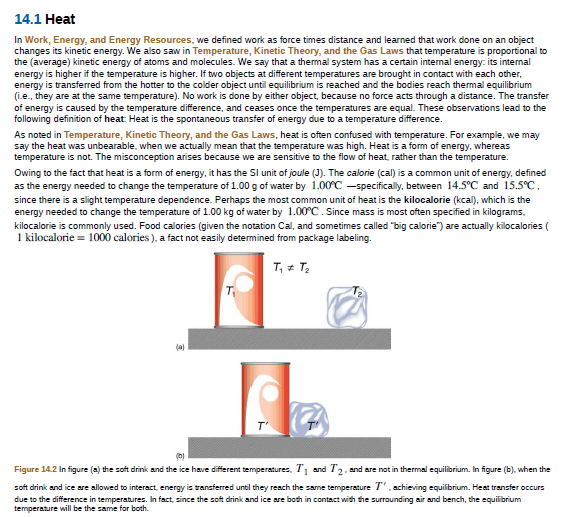
(a)
T'
Figure 14.2 In figure (a) the soft drinik and the ice have different temperatures, 71 and T2. and are not in thermal equilibrium. In figure (b), when the
soft drink and ice are allowed to interact, energy is transferred until they reach the same temperature /'.achieving equilibrium. Heat transfer occurs
due to the difference in temperatures. In fact, since the soft drink and ice are both in contact with the surrounding air and bench, the equilibrium
temperature will be the same for both.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning