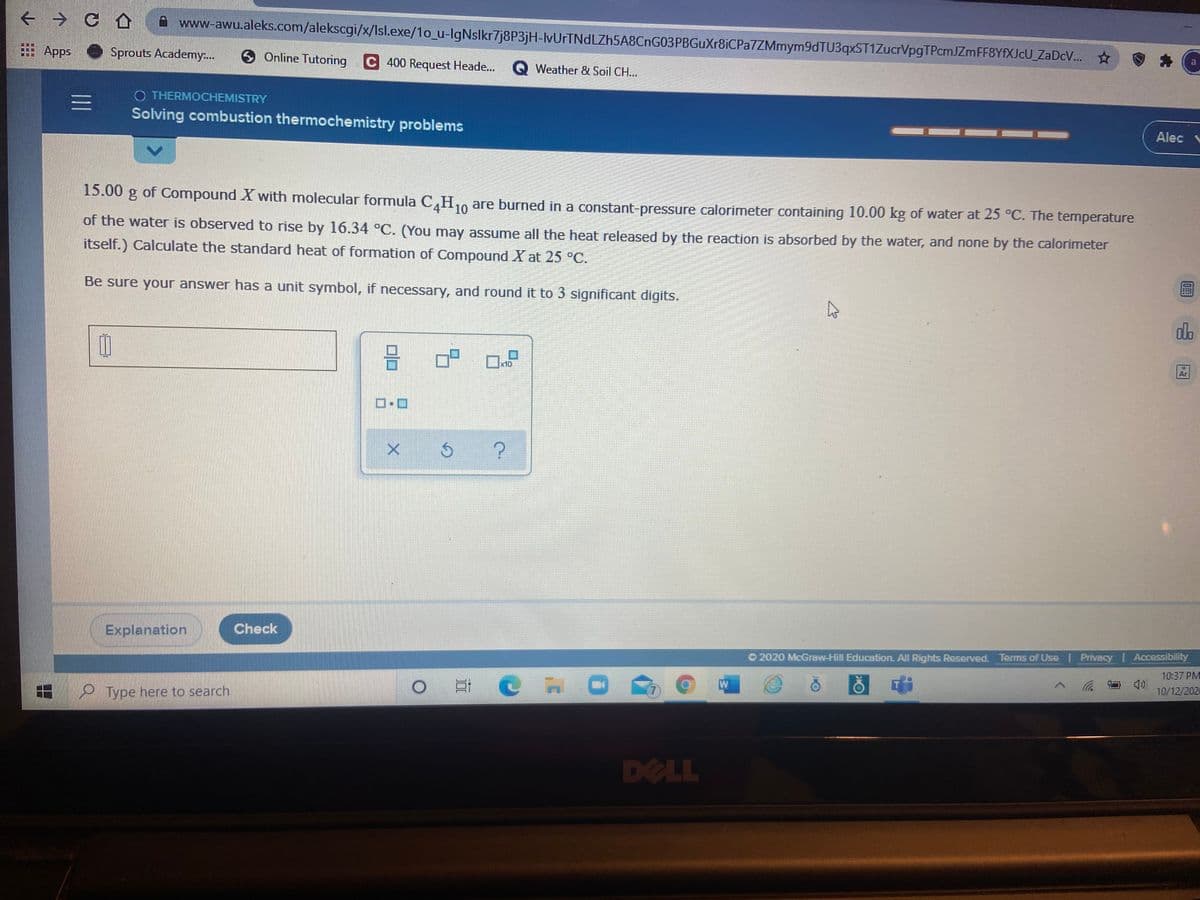
15.00 g of Compound X with molecular formula C,H10 are burned in a constant-pressure calorimeter containing 10.00 kg of water at 25 °C. The temperature of the water is observed to rise by 16.34 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound X at 25 °C. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
15.00 g of Compound X with molecular formula C,H10 are burned in a constant-pressure calorimeter containing 10.00 kg of water at 25 °C. The temperature of the water is observed to rise by 16.34 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound X at 25 °C. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
100%
Transcribed Image Text:www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8ICPa7ZMmym9dTU3qxST1ZucrVpgTPcmJZmFF8YfXJcU_ZaDcV...
Apps
Sprouts Academy:..
6 Online Tutoring
C 400 Request Heade...
Weather & Soil CH...
O THERMOCHEMISTRY
=
Solving combustion thermochemistry problems
Alec
15.00 g of Compound X with molecular formula C,H,, are burned in a constant-pressure calorimeter containing 10.00 kg of water at 25 °C. The temperature
10
of the water is observed to rise by 16.34 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter
itself.) Calculate the standard heat of formation of Compound X at 25 °C.
Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
x10
Ar
Explanation
Check
O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessibility
SEUETPEebe
10:37 PM
2 Type here to search
7)
W
10/12/2020
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning