1A 8A H 2A 3A 4A SA 6A 7A He BCNOF Ne Li Be 1B 2B ASi P S CI Ar Na Mg 3B 4B 58 6B 7B 88 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe Cs Ba La Hf Ta wRe Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd P Sm Eu 6d Tb Dy Ho Er Im Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr () What is the name of the element with a valence electron configuration of 4s24p4? (2) What is the name of the element with a valence electron configuration of 3s23p2?
1A 8A H 2A 3A 4A SA 6A 7A He BCNOF Ne Li Be 1B 2B ASi P S CI Ar Na Mg 3B 4B 58 6B 7B 88 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe Cs Ba La Hf Ta wRe Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd P Sm Eu 6d Tb Dy Ho Er Im Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr () What is the name of the element with a valence electron configuration of 4s24p4? (2) What is the name of the element with a valence electron configuration of 3s23p2?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and e xplain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
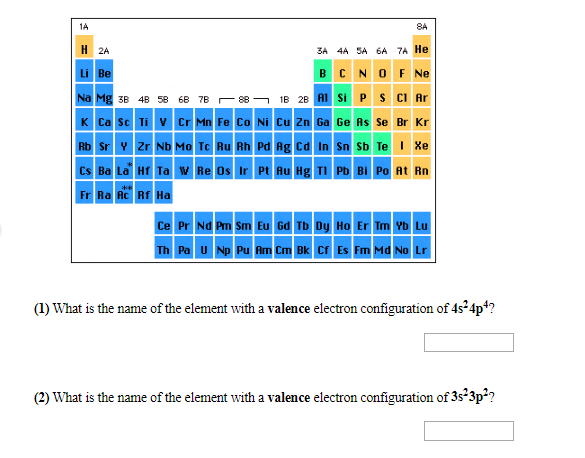
Transcribed Image Text:1A
8A
H 2A
3A 4A SA 6A 7A He
BCNOF Ne
Li Be
1B 2B ASi P S CI Ar
Na Mg 3B 4B 58 6B 7B 88
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe
Cs Ba La Hf Ta wRe Os Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd P Sm Eu 6d Tb Dy Ho Er Im Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
() What is the name of the element with a valence electron configuration of 4s24p4?
(2) What is the name of the element with a valence electron configuration of 3s23p2?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning