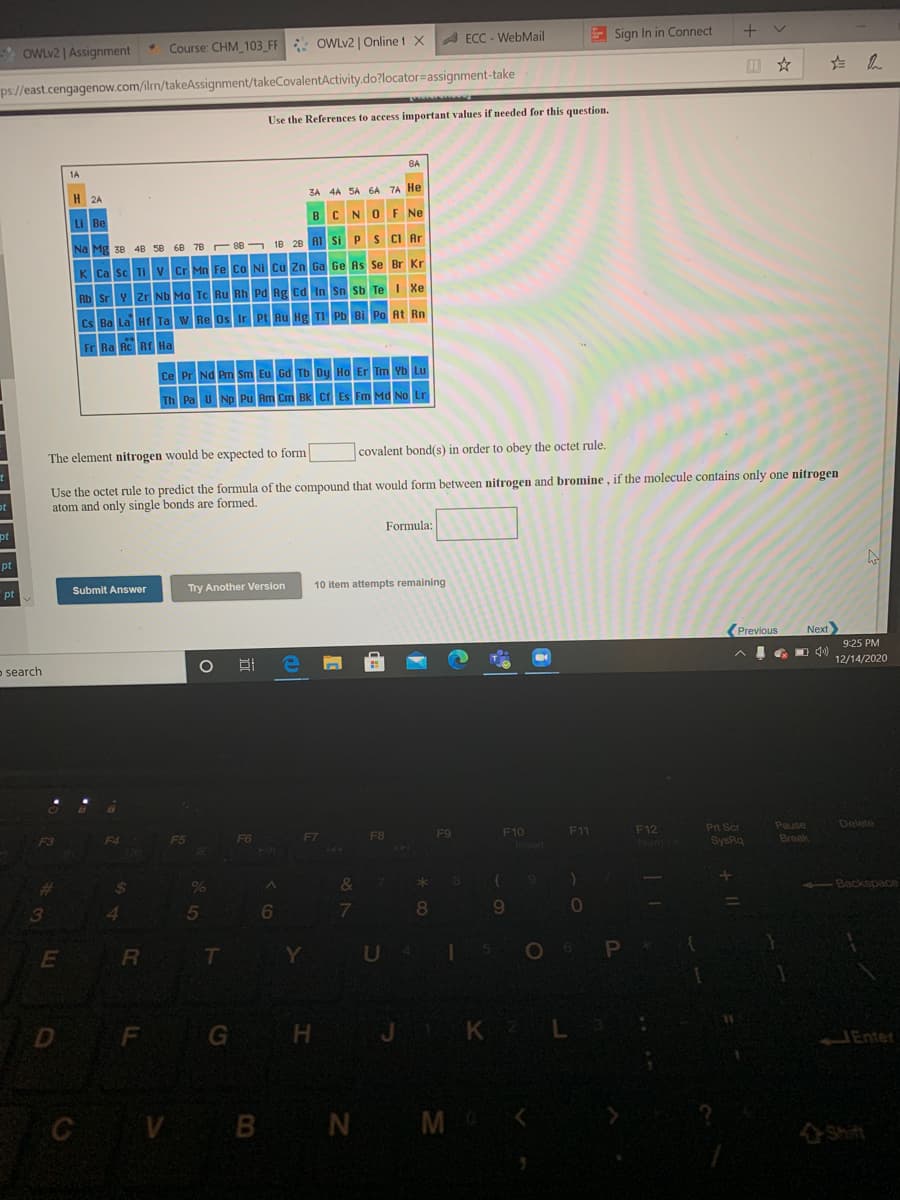
1A 8A H 2A 3A 4A SA 6A 7A He Li Be B. CNOF Ne Na Mg 38 4B 58 68 78 88 18 28 A1 Si P S CI Ar K Ca sc TI V Cr Mn Fe Co NI Cu Zn Ga Ge As Se Br Kr Rb Sr v zr Nb Mo To Ru Rh Pd Rg Cd In Sn Sb Te I Xe Cs Ba La H Ta w Re Os Ir Pt Au Hg TI Pb BI Po At Rn Fr Ra RC Rr Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Vb Lu Th Pa U Np Pu Am Cm Bk Cr Es Fm Md No Lr The element nitrogen would be expected to form |covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula of the compound that would form between nitrogen and bromine, if the molecule contains only one nitrogen atom and only single bonds are formed. Formula:
1A 8A H 2A 3A 4A SA 6A 7A He Li Be B. CNOF Ne Na Mg 38 4B 58 68 78 88 18 28 A1 Si P S CI Ar K Ca sc TI V Cr Mn Fe Co NI Cu Zn Ga Ge As Se Br Kr Rb Sr v zr Nb Mo To Ru Rh Pd Rg Cd In Sn Sb Te I Xe Cs Ba La H Ta w Re Os Ir Pt Au Hg TI Pb BI Po At Rn Fr Ra RC Rr Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Vb Lu Th Pa U Np Pu Am Cm Bk Cr Es Fm Md No Lr The element nitrogen would be expected to form |covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula of the compound that would form between nitrogen and bromine, if the molecule contains only one nitrogen atom and only single bonds are formed. Formula:
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.7QAP: A 3.03-g petroleum specimen was decomposed by wet ashing and subsequently diluted to 500 mL in a...
Related questions
Question
Transcribed Image Text:* OWLV2 | Assignment
Course: CHM_103 FF OWLV2 | Online 1 X
A ECC - WebMail
E Sign In in Connect
ps//east.cengagenow.com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take
Use the References to access important values if needed for this question.
8A
1A
H 2A
3A
4A SA 6A 7A He
BCNO F Ne
M38
18 28 Al Si P S CI Ar
68 7B
Ge As Se
At Rn
The element nitrogen would be expected to form
covalent bond(s) in order to obey the octet rule.
Use the octet rule to predict the formula of the compound that would form between nitrogen and bromine , if the molecule contains only one nitrogen
atom and only single bonds are formed.
Formula:
pt
pt
Submit Answer
Try Another Version
10 item attempts remaining
Previous
Next
9:25 PM
o search
D 4)
12/14/2020
Prn Scr
SysRq
F5
F6
F8
F9
F10
F11
F12
Pause
Delete
Num
Break
Backspace
4.
5
7
8
9.
Y
U
D F
G H J K L
Enter
C V B N M <
Shift
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning