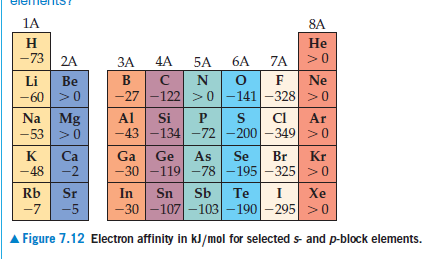
1A 8A н -73 Не 2A 6A 7A ЗА 5A B Ne Li Be -27 -122 >0 -141-328 >0 -60 Na Mg |-53 >0 Al CI Si Ar -43 -134 -72 -200 -349 >0 K -48 Ca -2 As Se -30 -119 -78 -195-325 >0 Ga Ge Br Kr Sr -5 Rb In Sn Sb Te Xe -30 -107-103–190 -295 >0 -7 A Figure 7.12 Electron affinity in kJ/mol for selected s- and p-block elements.
1A 8A н -73 Не 2A 6A 7A ЗА 5A B Ne Li Be -27 -122 >0 -141-328 >0 -60 Na Mg |-53 >0 Al CI Si Ar -43 -134 -72 -200 -349 >0 K -48 Ca -2 As Se -30 -119 -78 -195-325 >0 Ga Ge Br Kr Sr -5 Rb In Sn Sb Te Xe -30 -107-103–190 -295 >0 -7 A Figure 7.12 Electron affinity in kJ/mol for selected s- and p-block elements.
Chapter9: Energy For Today
Section: Chapter Questions
Problem 9E
Related questions
Question
Group 4A elements have much more negative electron
affinities than their neighbors in groups 3A and 5A (see
Figure 7.12). Which of the following statements best explains
this observation? (i) The group 4A elements have much higher
first ionization energies than their neighbors in groups 3A
and 5A. (ii) The addition of an electron to a group 4A element
leads to a half-filled np3 outer electron configuration.
(iii) The group 4A elements have unusually large atomic radii.
(iv) The group 4A elements are easier to vaporize than are
the group 3A and 5A elements.
Transcribed Image Text:1A
8A
н
-73
Не
2A
6A
7A
ЗА
5A
B
Ne
Li
Be
-27 -122 >0
-141-328 >0
-60
Na Mg
|-53 >0
Al
CI
Si
Ar
-43 -134 -72 -200 -349 >0
K
-48
Ca
-2
As
Se
-30 -119 -78 -195-325 >0
Ga
Ge
Br
Kr
Sr
-5
Rb
In
Sn
Sb
Te
Xe
-30 -107-103–190 -295 >0
-7
A Figure 7.12 Electron affinity in kJ/mol for selected s- and p-block elements.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning