2) a) Consider the following molecule hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. Given what you have learned about b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the which group. HS
2) a) Consider the following molecule hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. Given what you have learned about b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the which group. HS
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 66SCQ: Lets look more closely at the process of hybridization. (a) What is the relationship between the...
Related questions
Question

Transcribed Image Text:2) a) Consider the following molecule
hybridization theory, draw an image or images explaining the bonding situation in this
molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap
to form the bonds in the molecule. Indicate the % s or p character in the given atomic and
hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain
the image or images you just drew.
Given what you have learned about
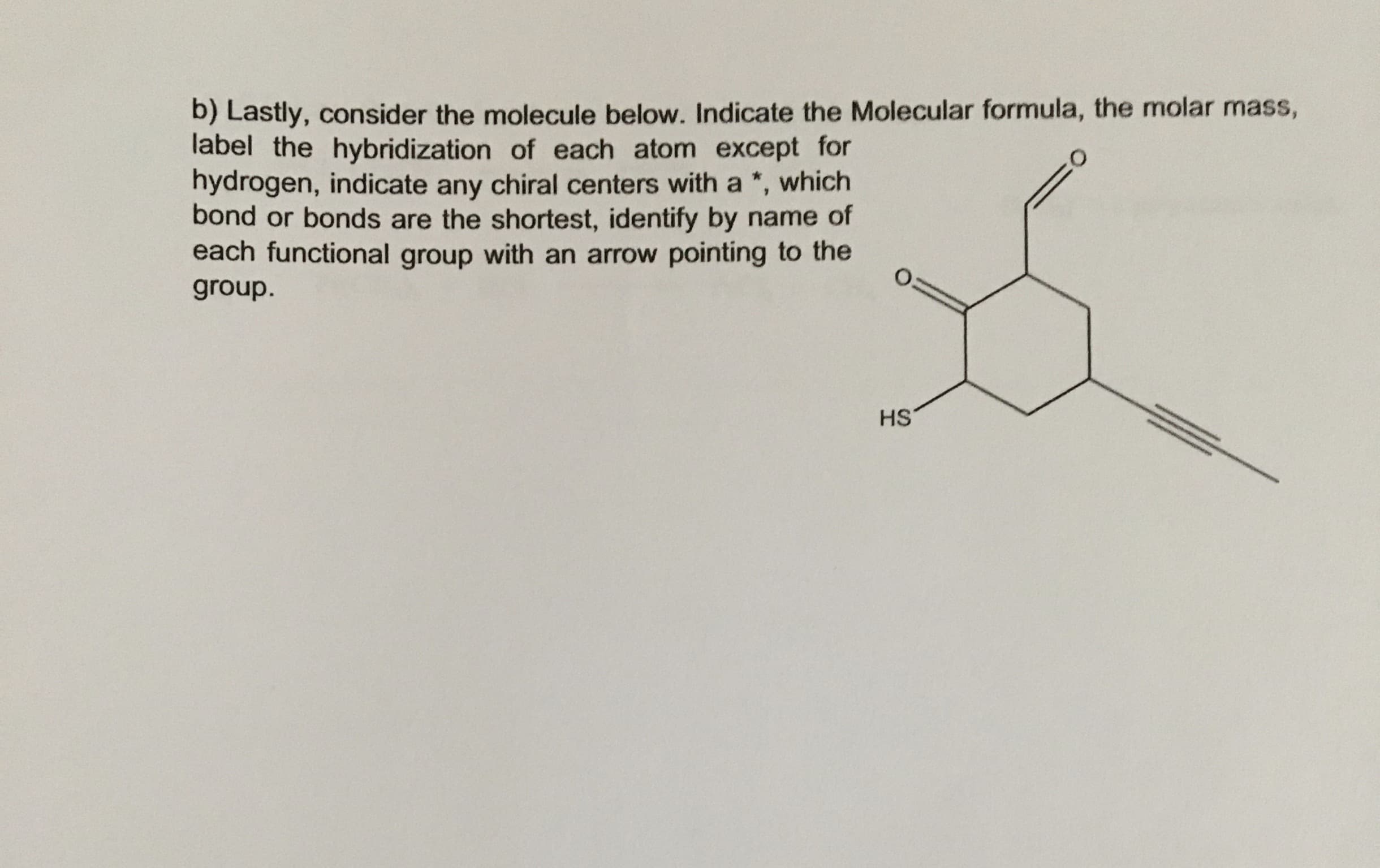
Transcribed Image Text:b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass,
label the hybridization of each atom except for
hydrogen, indicate any chiral centers with a *,
bond or bonds are the shortest, identify by name of
each functional group with an arrow pointing to the
which
group.
HS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
