2) Which of the following sets of quantum numbers is allowed? a. n=2, { = 1, m, = +1/2, ms=-1/2 b. n= 3, { = 2, m =+1, m, = +1 c. n= 4, { = 1,, — 0, т,—-1/2 me d. n=4, { = 3, =-1, m, = 0 %3D е. п%3D5, %3D2, = +2, ms=-1
2) Which of the following sets of quantum numbers is allowed? a. n=2, { = 1, m, = +1/2, ms=-1/2 b. n= 3, { = 2, m =+1, m, = +1 c. n= 4, { = 1,, — 0, т,—-1/2 me d. n=4, { = 3, =-1, m, = 0 %3D е. п%3D5, %3D2, = +2, ms=-1
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 13CO: • identify an orbital (as 1s, 3p, etc.) from its quantum numbers, or vice versa.
Related questions
Question
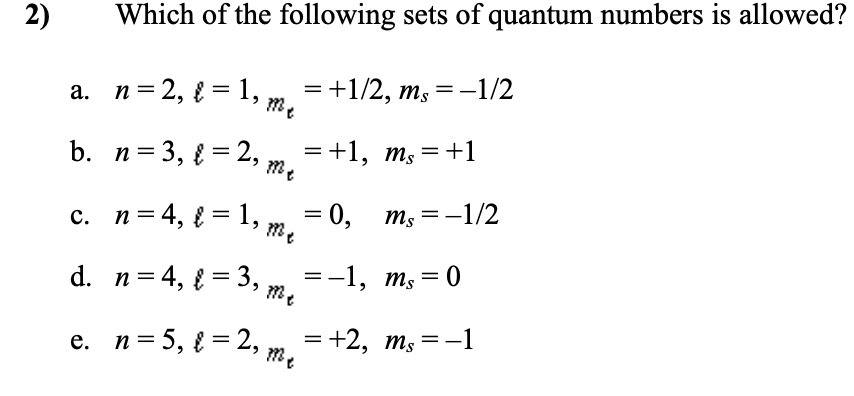
Transcribed Image Text:n= 2, { = 1, m,
2)
Which of the following sets of quantum numbers is allowed?
а. п%3D2, 3D 1,
= +1/2, ms=-1/2
b. n=3, { = 2, ,
= +1, m, =+1
c. n= 4, { = 1, m
3D 0, тs
m; =-1/2
d. n= 4, { = 3,
=-1, ms = 0
e. n= 5, { = 2,
= +2, m,=-1
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning