2. a) How many moles of C0, are in 68.0 g of CO,? Jeam O1OH 1o salom vasnm b) How many atoms of potassium are in 0.150 moles of potassium? 0.08 di Ledmeg to courbrere ODH lo c) How many moles of Cl, are in 5000 molecules of Cl,? CIA To
2. a) How many moles of C0, are in 68.0 g of CO,? Jeam O1OH 1o salom vasnm b) How many atoms of potassium are in 0.150 moles of potassium? 0.08 di Ledmeg to courbrere ODH lo c) How many moles of Cl, are in 5000 molecules of Cl,? CIA To
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter33: Preparation Of Copper(i) Chloride
Section: Chapter Questions
Problem 5ASA
Related questions
Question
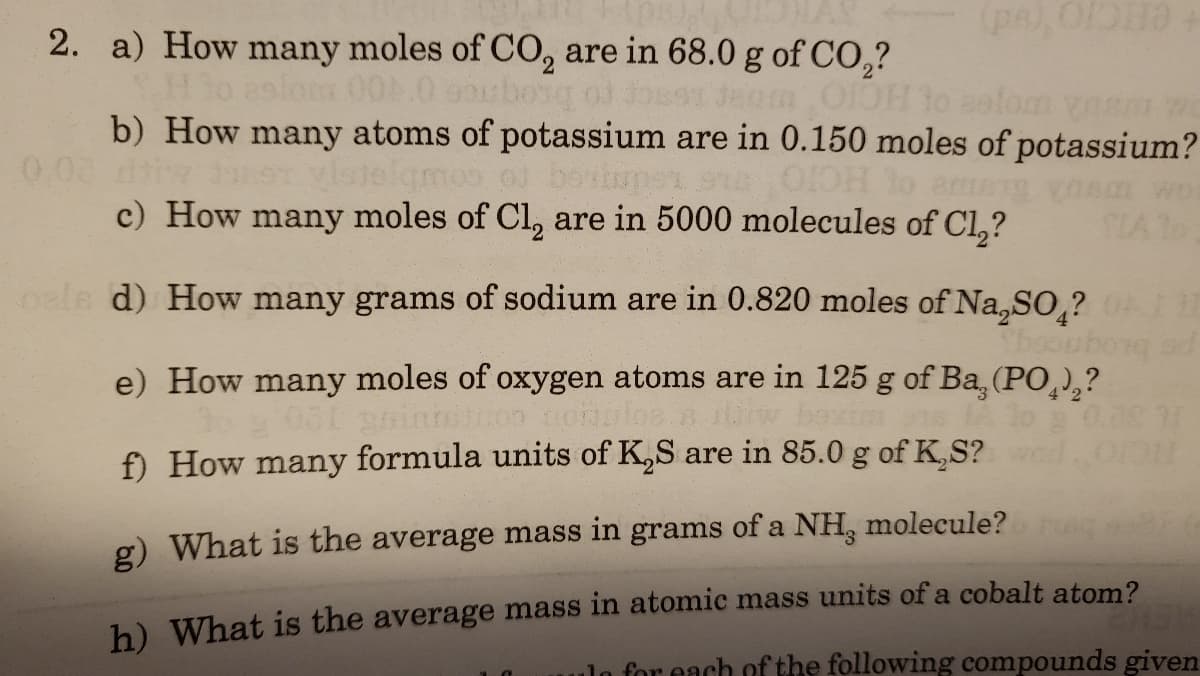
Transcribed Image Text:(pe), ODH
2. a) How many moles of CO, are in 68.0 g of CO,?
29lom 00.0 spbong od for
b) How many atoms of potassium are in 0.150 moles of potassium?
o boviupen s
c) How many moles of Cl, are in 5000 molecules of Cl,?
0.08
9T vlotelgmos
HCIO
amg vonm wo
SLA To
oale d) How many grams of sodium are in 0.820 moles of Na,SO ?
e) How many moles of oxygen atoms are in 125 g of Ba, (PO,),?
4 2
f) How many formula units of K,S are in 85.0 g of K,S?
ICIO
g) What is the average mass in grams of a NH, molecule?
h) What is the average mass in atomic mass units of a cobalt atom?
ulo for each of the following compounds given
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning