2. Complete the following table by supplying the missing temperatures in each row. Be certain to retain the appropriate number of significant figures in each case. Units Kelvin (K) Celcius (°C) Fahrenheit (°F) b) 35.68 a) d) c) Temperature 72.5 461 f) e) Show Calculations Below c) ib) a) f) e) d) 3. For each of the following problems, use equivalence statements to develop a "roadmap" from the given information to the desired outcome. provide the equivalence statements necessary to do so. DO NOT SOLVE THE PROBLEMS - simply a) How nanoseconds are equivalent to 2.86 x 10 minutes? GIVEN EQUIVALENCE STATEMENT(S) DESIRED 2.86 x 10-6 minutes ? nanoseconds b) The distance between the centers of the two atoms in an oxygen molecule is 1.21 x 10- meters. Calculate this distance in inches GIVEN DESIRED EQUIVALENCE STATEMENT(S) 1.21 x 10-10 m ? inch c) The 1500. meter race is sometimes referred to as the "metric mile". Express this distance in miles. GIVEN DESIRED EQUIVALENCE STATEMENT(S) 1500. m ? mile
2. Complete the following table by supplying the missing temperatures in each row. Be certain to retain the appropriate number of significant figures in each case. Units Kelvin (K) Celcius (°C) Fahrenheit (°F) b) 35.68 a) d) c) Temperature 72.5 461 f) e) Show Calculations Below c) ib) a) f) e) d) 3. For each of the following problems, use equivalence statements to develop a "roadmap" from the given information to the desired outcome. provide the equivalence statements necessary to do so. DO NOT SOLVE THE PROBLEMS - simply a) How nanoseconds are equivalent to 2.86 x 10 minutes? GIVEN EQUIVALENCE STATEMENT(S) DESIRED 2.86 x 10-6 minutes ? nanoseconds b) The distance between the centers of the two atoms in an oxygen molecule is 1.21 x 10- meters. Calculate this distance in inches GIVEN DESIRED EQUIVALENCE STATEMENT(S) 1.21 x 10-10 m ? inch c) The 1500. meter race is sometimes referred to as the "metric mile". Express this distance in miles. GIVEN DESIRED EQUIVALENCE STATEMENT(S) 1500. m ? mile
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 73QAP: A Different civilization on a distant planet has developed a new temperature scale based on ethyl...
Related questions
Question
Need help
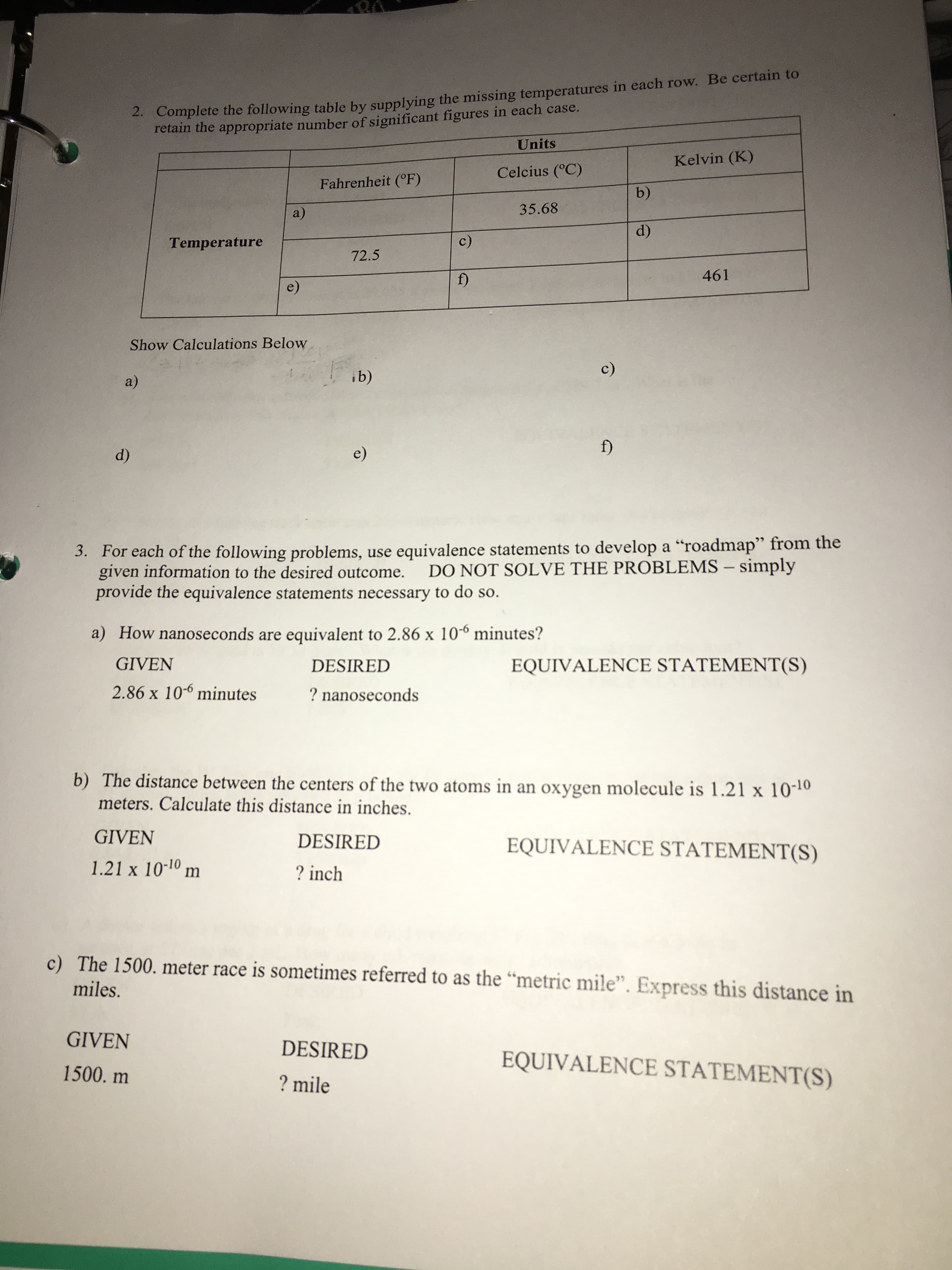
Transcribed Image Text:2. Complete the following table by supplying the missing temperatures in each row. Be certain to
retain the appropriate number of significant figures in each case.
Units
Kelvin (K)
Celcius (°C)
Fahrenheit (°F)
b)
35.68
a)
d)
c)
Temperature
72.5
461
f)
e)
Show Calculations Below
c)
ib)
a)
f)
e)
d)
3. For each of the following problems, use equivalence statements to develop a "roadmap" from the
given information to the desired outcome.
provide the equivalence statements necessary to do so.
DO NOT SOLVE THE PROBLEMS - simply
a) How nanoseconds are equivalent to 2.86 x 10
minutes?
GIVEN
EQUIVALENCE STATEMENT(S)
DESIRED
2.86 x 10-6 minutes
? nanoseconds
b) The distance between the centers of the two atoms in an oxygen molecule is 1.21 x 10-
meters. Calculate this distance in inches
GIVEN
DESIRED
EQUIVALENCE STATEMENT(S)
1.21 x 10-10 m
? inch
c) The 1500. meter race is sometimes referred to as the "metric mile". Express this distance in
miles.
GIVEN
DESIRED
EQUIVALENCE STATEMENT(S)
1500. m
? mile
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning



Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning