2. Consider the following pair of reactions: 2 B (desired) 2 C (undesired) A A Suppose 100 mol of A is fed to a batch reactor and the final product contains 10 mol of A, 160 mol 0f B, and 10 mol of C. Calculate the following: a. The fraction conversion of A b. The percentage yield of B. c. The selectivity of B relative to C. d. The extents of first and second reactions.
2. Consider the following pair of reactions: 2 B (desired) 2 C (undesired) A A Suppose 100 mol of A is fed to a batch reactor and the final product contains 10 mol of A, 160 mol 0f B, and 10 mol of C. Calculate the following: a. The fraction conversion of A b. The percentage yield of B. c. The selectivity of B relative to C. d. The extents of first and second reactions.
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter8: Thin-layer Chromatography: Analyzing Analgesics And Isolating Lycopene From Tomato Paste
Section: Chapter Questions
Problem 10Q: A TLC plate showed two spots with Rf values of 0.25 and 0.26. The plate was removed from the...
Related questions
Question
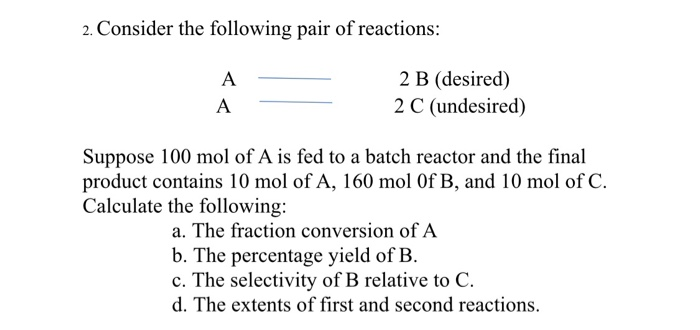
Transcribed Image Text:2. Consider the following pair of reactions:
2 B (desired)
2 C (undesired)
A
A
Suppose 100 mol of A is fed to a batch reactor and the final
product contains 10 mol of A, 160 mol Of B, and 10 mol of C.
Calculate the following:
a. The fraction conversion of A
b. The percentage yield of B.
c. The selectivity of B relative to C.
d. The extents of first and second reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole